
Organic Chemistry
12th Edition
ISBN: 9781118875766
Author: T. W. Graham Solomons, Craig B. Fryhle, Scott A. Snyder
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 13, Problem 44P
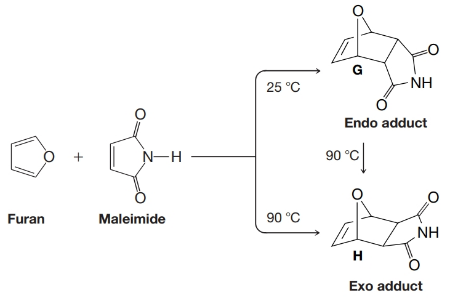
When furan and maleimide undergo a Diels–Alder reaction at 25°C, the major product is the endo adduct G. When the reaction is carried out at 90°C, however, the major product is the exo isomer H. The endo adduct isomerizes to the exo adduct when it is heated to 90°C. Propose an explanation that will account for these results.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Please answer both parts to the questions completely. I will rate the answer afterwards.
For a diels-alder reaction between anthracene and maleic anhydride, are the exo and endo forms of product 9,10-dihydroanthracene-9,10-ɑ,β-succinic acid anhydride different stereoisomers or are they the same molecule? Explain your answer by drawing the molecule.
Would it be favorable to get a 1,4-adduct of anthracene and maleic anhydride? Why or why not? If the 1,4-adduct of anthracene and maleic anhydride had formed, would it have different exo and endo isomers? Yes or no and why?
what is the product after these compumds undergo a diels-alder reaction
Diels–Alder reaction of a monosubstituted diene (such as CH2=CH–CH=CHOCH3) with a monosubstituted dienophile (such as CH2=CHCHO)gives a mixture of products, but the 1,2-disubstituted product oftenpredominates. Draw the resonance hybrid for each reactant, and use thecharge distribution of the hybrids to explain why the 1,2-disubstitutedproduct is the major product.
Chapter 13 Solutions
Organic Chemistry
Ch. 13 - Prob. 1PPCh. 13 - Prob. 2PPCh. 13 - Prob. 3PPCh. 13 - Practice Problem 13.4 From each set of resonance...Ch. 13 - Practice Problem 13.5 The following enol (an...Ch. 13 - Prob. 6PPCh. 13 - Practice Problem 13.7
Two compounds, A and B, have...Ch. 13 - Prob. 8PPCh. 13 - Prob. 9PPCh. 13 - Prob. 10PP
Ch. 13 - Prob. 11PPCh. 13 - Prob. 12PPCh. 13 - Prob. 13PPCh. 13 - Prob. 14PPCh. 13 - Prob. 15PPCh. 13 - Practice Problem 13.16
Diels–Alder reactions also...Ch. 13 - Prob. 17PPCh. 13 - Prob. 18PCh. 13 - What product would you expect from the following...Ch. 13 - Prob. 20PCh. 13 - Prob. 21PCh. 13 - Provide the reagents necessary for each of the...Ch. 13 - Prob. 23PCh. 13 - Prob. 24PCh. 13 - Prob. 25PCh. 13 - When 1-pentene reacts with N-bromosuccinimide...Ch. 13 - Prob. 27PCh. 13 - Prob. 28PCh. 13 - Prob. 29PCh. 13 - Prob. 30PCh. 13 - 13.31 Provide a mechanism that explains formation...Ch. 13 - 13.32 Provide a mechanism that explains formation...Ch. 13 - Treating either 1-chloro-3-methyl-2-butene or...Ch. 13 - Prob. 34PCh. 13 - Prob. 35PCh. 13 - Although both 1-bromobutane and 4-bromo-1-butene...Ch. 13 - Prob. 37PCh. 13 - Prob. 38PCh. 13 - Prob. 39PCh. 13 - Prob. 40PCh. 13 - Prob. 41PCh. 13 - Prob. 42PCh. 13 - Prob. 43PCh. 13 - 13.44 When furan and maleimide undergo a...Ch. 13 - Two controversial hard insecticides are aldrin and...Ch. 13 - Prob. 46PCh. 13 - Prob. 47PCh. 13 - Prob. 48PCh. 13 - Prob. 49PCh. 13 - Prob. 50PCh. 13 - Explain the product distribution below based on...Ch. 13 - Mixing furan (Problem 13.44) with maleic anhydride...Ch. 13 - Prob. 53PCh. 13 - Prob. 54PCh. 13 - Prob. 1LGPCh. 13 - Prob. 2LGP
Additional Science Textbook Solutions
Find more solutions based on key concepts
Determine [OH], [H+], and the pH of each of the following solutions. a. 1.0 M KCl b. 1.0 M KC2H3O2
Chemistry
8.63 Two flasks of equal volume and at the same temperature contain different gases. One flask contains 10.0 g ...
Chemistry: An Introduction to General, Organic, and Biological Chemistry (12th Edition) - Standalone book
Q4. Which property of rubbing alcohol is a chemical property?
a) Density (0.786 g/cm3)
b) Flammability
c) Bo...
Chemistry: A Molecular Approach (4th Edition)
Ethanol can be produced commercially by the hydration of ethylene: C2H4 + H2O -* C2H5OH Some of the product is ...
Elementary Principles of Chemical Processes, Binder Ready Version
Rank the given solvents in decreasing order of their ability to dissolve each compound. Solutes
Organic Chemistry (9th Edition)
4. 38 Strontium has four naturally occurring isotopes, with mass numbers 84, 86, 87, arid 88.
a. Write the atom...
Basic Chemistry (5th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The following triene undergoes an intramolecular Diels-Alder reaction to give a bicyclic product. Propose a structural formula for the product. Account for the observation that the Diels-Alder reaction given in this problem takes place under milder conditions (at lower temperature) than the analogous Diels-Alder reaction shown in Problem 20.34.arrow_forwardBASED ON THIS EXPERIMENT: The purpose of this experiment is to perform a Diels-Alder reaction between E,E-2,4-hexadien-1-ol and maleic anhydride in a "neat" or solventless condition. The Diels-Alder reaction is a [4+2] cycloaddition that forms a six-membered ring, typically a derivative of cyclohexene. The diene, E,E-2,4-hexadien-1-ol, and the dienophile, maleic anhydride, will be mixed and ground together, resulting in a eutectic mixture. The reaction is unique as it occurs without a solvent, contributing to a "greener" experiment. After the reaction, the product will be analyzed through mass measurements, and the mechanism involves a subsequent nucleophilic acyl substitution. QUESTION: how many carbon atoms does a dienophile contribute to the Diels-Alderadduct? How many carbon atoms does a diene contribute? draw andlabel which reagent is the diene, and which is the dienophile.arrow_forwardA2 What is the inverse electron demand Diels–Alder reaction? Please pick a pair of a diene and a dienophile from the following dienes and dienophile that will undergo this type of reaction. Please show how this reaction works using Frontier Molecular Orbitals. What is the reaction product?arrow_forward
- Write a general rule that can be used to predict the major product of a Diels–Alder reaction between an alkene with an electron-withdrawing substituent and a diene with a substituent that can donate electrons by resonance depending on the location of the substituent on the diene.arrow_forwardDiels–Alder reaction of a monosubstituted diene (such as CH2=CH– CH=CHOCH3) with a monosubstituted dienophile (such as CH2=CHCHO) gives a mixture of products, but the 1,2-disubstituted product often predominates. Draw the resonance hybrid for each reactant, and use the charge distribution of the hybrids to explain why the 1,2-disubstituted product is the major product.arrow_forwardDiels—Alder reaction of a monosubstituted diene (such as CH2 = CH – CH = CHOCH3) with a monosubstituted dienophile (such as CH2 = CHCHO) gives a mixture of products, but the 1,2-disubstituted product often predominates. Draw the resonance hybrid for each reactant and use the charge distribution of the hybrids to explain why the 1,2-disubstituted product is the major product.arrow_forward
- The Diels-Alder reaction is not limited to making six-membered rings with only carbon atoms. Predict the products of the following reaction that produce rings with atoms other than carbon in them.arrow_forwardThe following compound can carry out the Diels-Alder reactionarrow_forwardWhich constitutional isomer represents the product of this Diels-Alder reaction?arrow_forward
- Complete the Diels-Alder reactions by drawing structures for the products in each question.arrow_forwardFor a diels-alder reaction between anthracene and maleic anhydride, are the exo and endo forms of product 9,10-dihydroanthracene-9,10-ɑ,β-succinic acid anhydride different stereoisomers or are they the same molecule?arrow_forwardUemura and coworkers studied a time dependent Diels-Alder reaction which first formed the endo product as the major organic product and with time produced the exo product (J. Org. Chem. 2018, 83, 9300−9304). Show the endo and exo product for the reaction below. Which is the thermodynamic product and which is the kinetic product? Explain your reasoning.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

IR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=_TmevMf-Zgs;License: Standard YouTube License, CC-BY