
Concept explainers
(a)
Interpretation:
Whether crotonate, a C4 species is a (1) hydroxy acid, (2) keto acid, (3) saturated acid, or (4) unsaturated acid has to be identified.
Concept introduction:
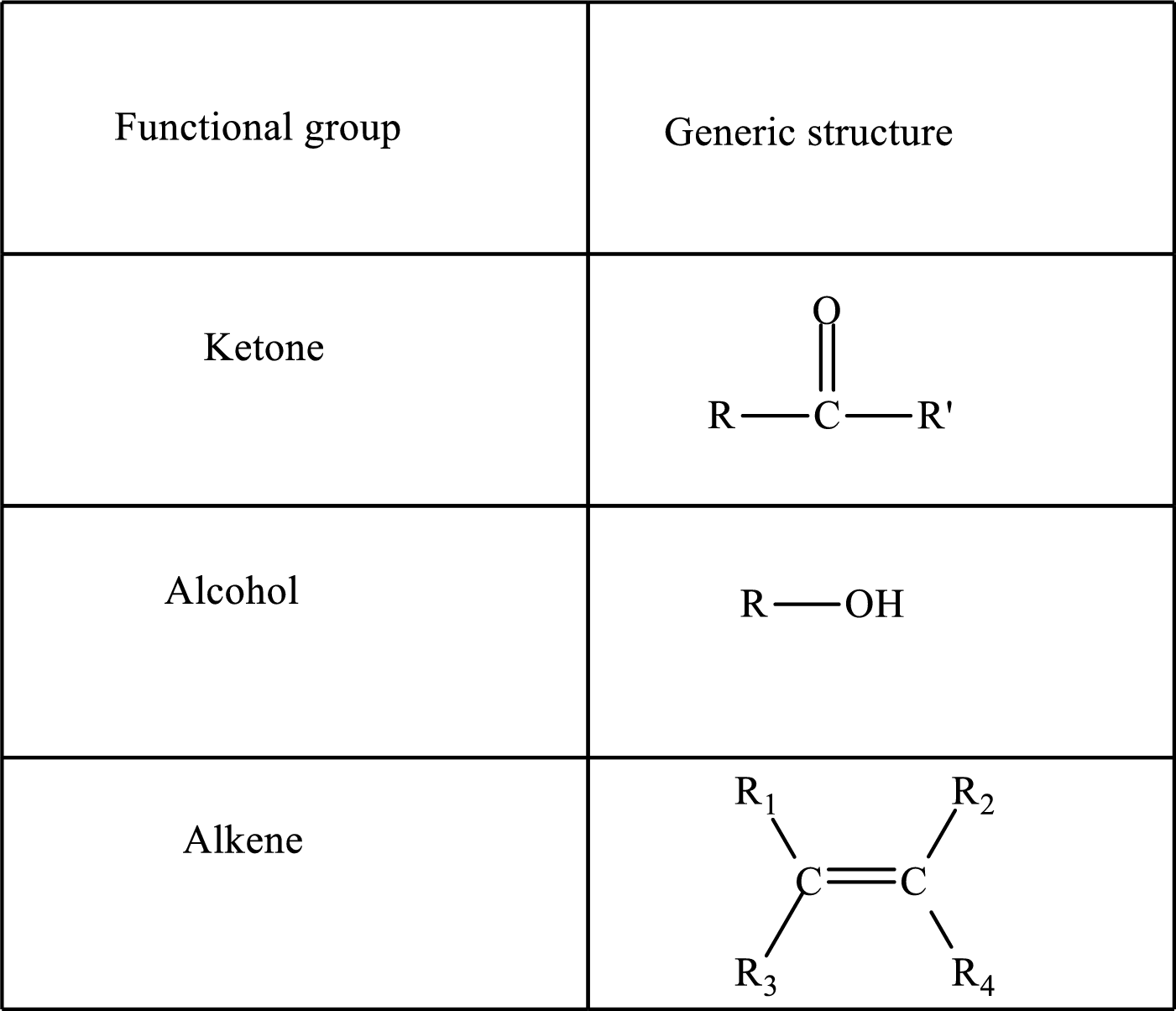
Here, R and R’ represent an alkyl group. In alkene, R1, R2, R3, and R4 can be the same or different or can be hydrogen.
Keto acid has a
A carboxylate group is formed by the removal of the acidic hydrogen from the carboxylic group. The conjugate base is formed by the removal of acidic hydrogen from the corresponding acid.
(b)
Interpretation:
Whether oxaloacetate, a C4 species is a (1) hydroxy acid, (2) keto acid, (3) saturated acid, or (4) unsaturated acid has to be identified.
Concept introduction:
Functional groups are defined as the group of atoms which are attached to the carbon backbone of organic compounds. These are generally heteroatoms which are attached to the parent hydrocarbon chain. Some examples of functional groups are as follows:
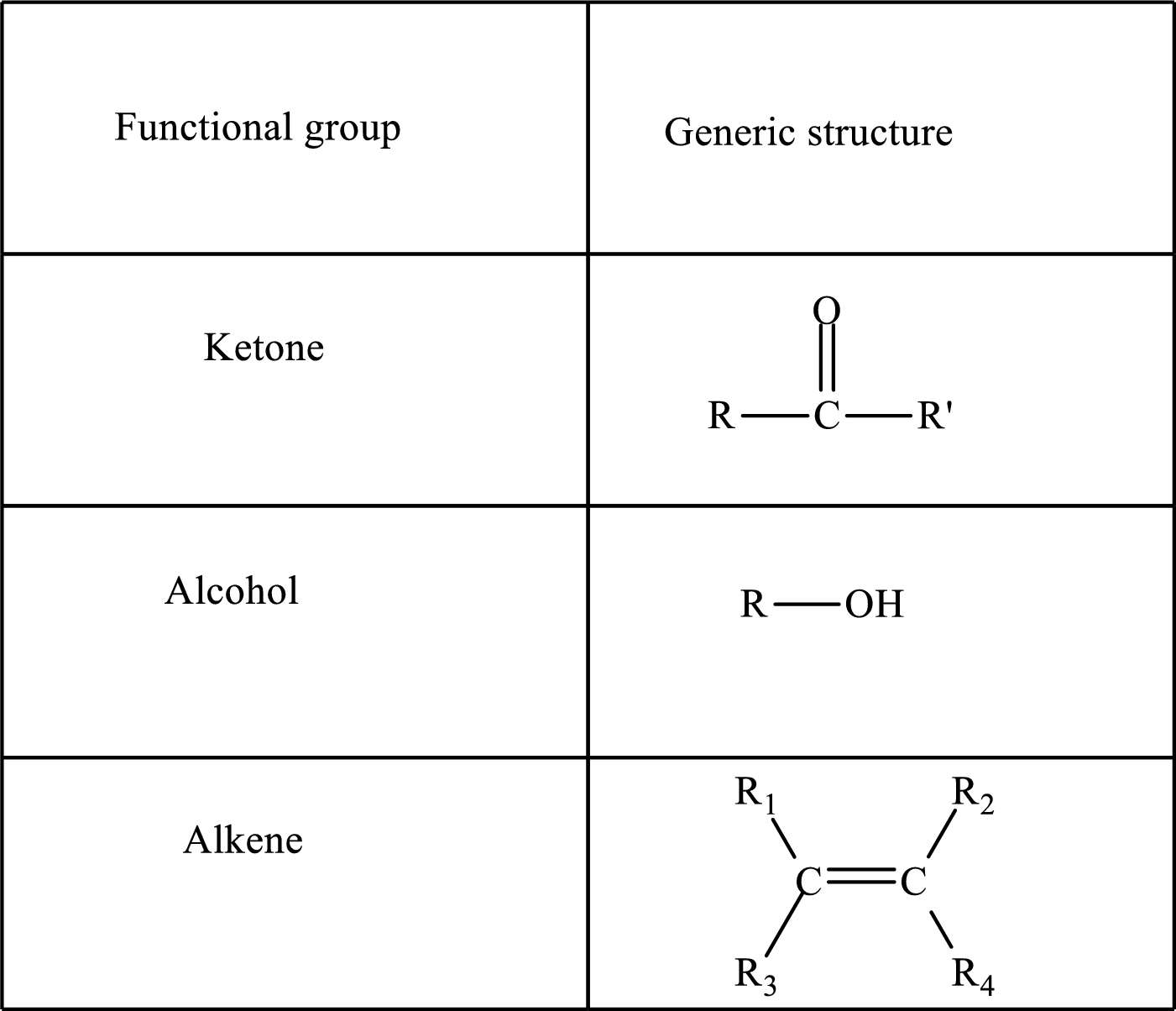
Here, R and R’ represent an alkyl group. In alkene, R1, R2, R3, and R4 can be the same or different or can be hydrogen. Alkanes are saturated hydrocarbons that contain covalently bonded hydrogen and carbon atoms. Alkenes have a double bond, hence; they are unsaturated compounds.
Keto acid has a ketone and a carboxylic acid (-COOH) group. Hydroxy acid has a hydroxy (-OH) group and a carboxylic acid (-COOH) group. Saturated acids contain single bonds between carbon atoms and a carboxylic group. Unsaturated acid contains a double or triple bond between carbon atoms and a carboxylic group.
A carboxylate group is formed by the removal of the acidic hydrogen from the carboxylic group. The conjugate base is formed by the removal of acidic hydrogen from the corresponding acid.
(c)
Interpretation:
Whether acetoacetate, a C4 species is a (1) hydroxy acid, (2) keto acid, (3) saturated acid, or (4) unsaturated acid has to be identified.
Concept introduction:
Functional groups are defined as the group of atoms which are attached to the carbon backbone of organic compounds. These are generally heteroatoms which are attached to the parent hydrocarbon chain. Some examples of functional groups are as follows:
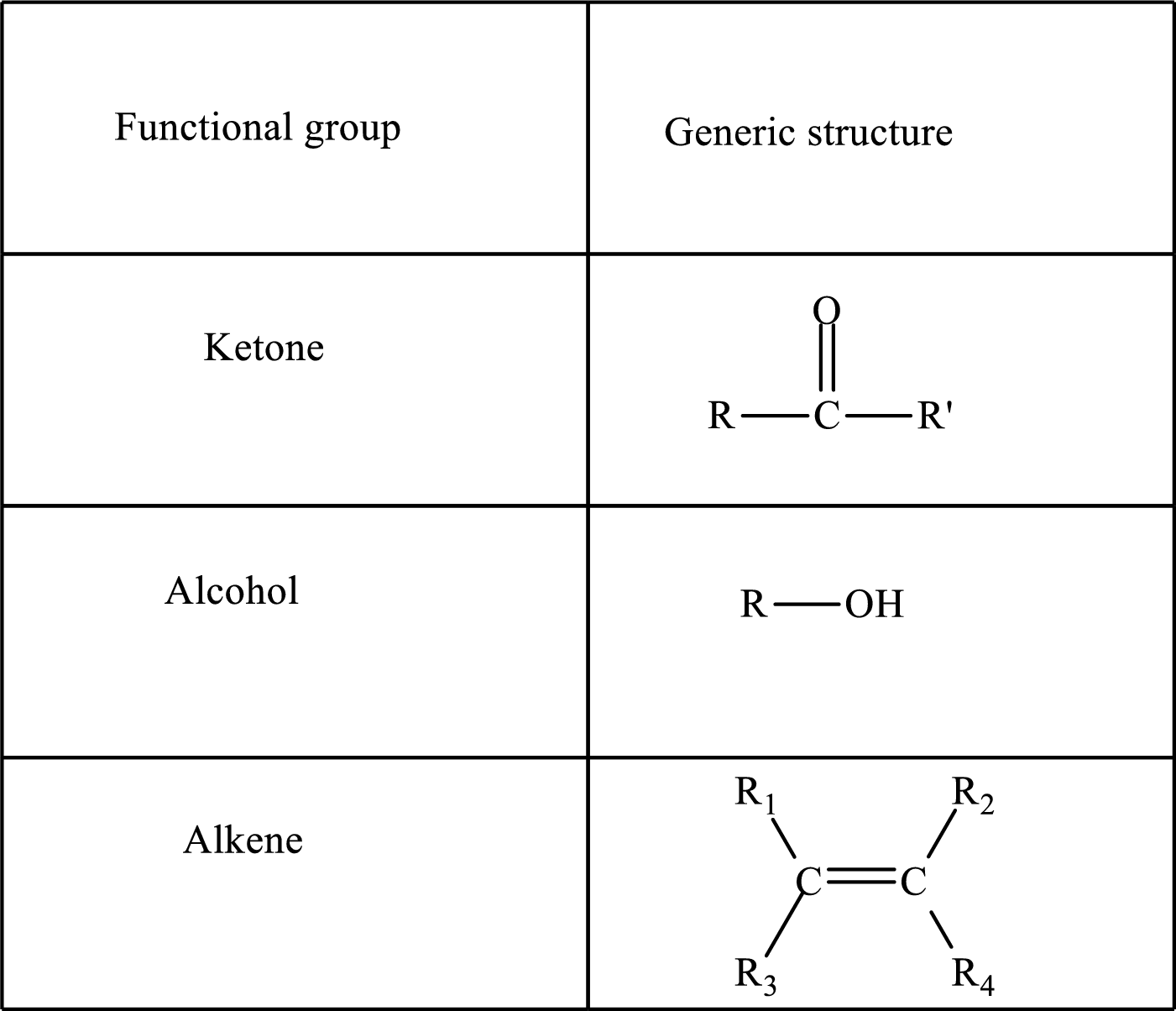
Here, R and R’ represent an alkyl group. In alkene, R1, R2, R3, and R4 can be the same or different or can be hydrogen. Alkanes are saturated hydrocarbons that contain covalently bonded hydrogen and carbon atoms. Alkenes have a double bond, hence; they are unsaturated compounds.
Keto acid has a ketone and a carboxylic acid (-COOH) group. Hydroxy acid has a hydroxy (-OH) group and a carboxylic acid (-COOH) group. Saturated acids contain single bonds between carbon atoms and a carboxylic group. Unsaturated acid contains a double or triple bond between carbon atoms and a carboxylic group.
A carboxylate group is formed by the removal of the acidic hydrogen from the carboxylic group. The conjugate base is formed by the removal of acidic hydrogen from the corresponding acid.
(d)
Interpretation:
Whether malate, a C4 species is a (1) hydroxy acid, (2) keto acid, (3) saturated acid, or (4) unsaturated acid has to be identified.
Concept introduction:
Functional groups are defined as the group of atoms which are attached to the carbon backbone of organic compounds. These are generally heteroatoms which are attached to the parent hydrocarbon chain. Some examples of functional groups are as follows:
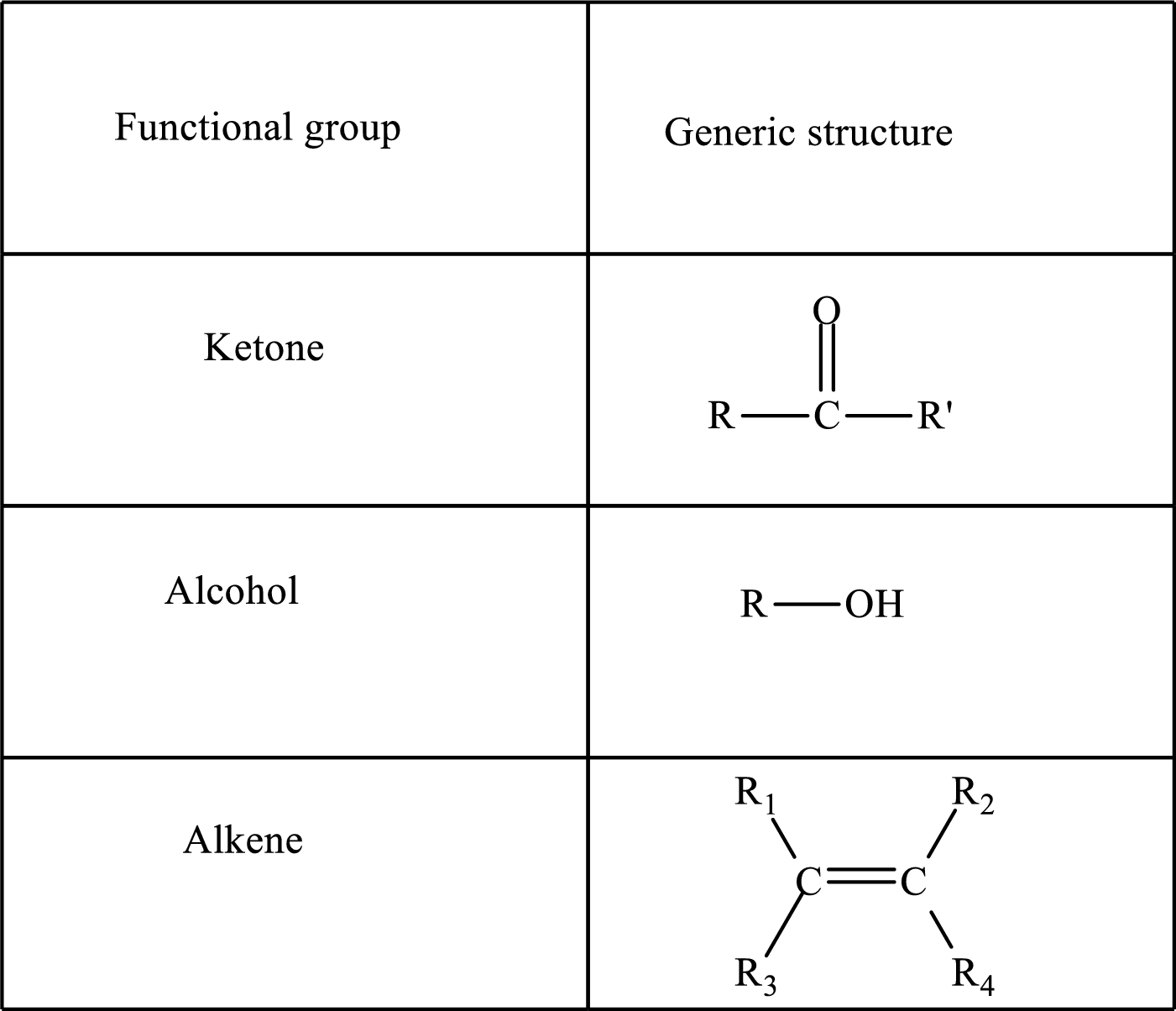
Here, R and R’ represent an alkyl group. In alkene, R1, R2, R3, and R4 can be the same or different or can be hydrogen. Alkanes are saturated hydrocarbons that contain covalently bonded hydrogen and carbon atoms. Alkenes have a double bond, hence; they are unsaturated compounds.
Keto acid has a ketone and a carboxylic acid (-COOH) group. Hydroxy acid has a hydroxy (-OH) group and a carboxylic acid (-COOH) group. Saturated acids contain single bonds between carbon atoms and a carboxylic group. Unsaturated acid contains a double or triple bond between carbon atoms and a carboxylic group.
A carboxylate group is formed by the removal of the acidic hydrogen from the carboxylic group. The conjugate base is formed by the removal of acidic hydrogen from the corresponding acid.
Want to see the full answer?
Check out a sample textbook solution
Chapter 14 Solutions
Organic And Biological Chemistry
- Egg yolk contains a lot of lecithin (a phosphoglyceride). After ingesting a hard-boiled egg, would you find an increase in the lecithin level of your blood? Explain.arrow_forward2. For the chemical reaction that results in the formation of a peptide bond between two amino acids, G = +4 kcal/mol. Based on this information, which of the following labels would be appropriate to describe this reaction? Exergonic Hydrolysis Redox Endergonicarrow_forwarddraw the structure of CaCO3arrow_forward
- WHAT WAS THE UREASE TEST ORIGINALLY DESIGNED FOR? WHAT ORGANISM IS USED TI DEMONSTRATE A UREASE POSITIVE TEST? WHAT IS THE INDICATOR IN THE UREASE TEST AND WHY WAS IT USED? WHAT IS THE COLOR OF A POSITIVE TEST AND WHY IS IT THAR COLOR? WHAT DOES A ORANGE-YELLOW COLOR AT THE END OF 24 HOURS REPRESENT? IF THIS SAME TUBE PRODUCED A PINK COLOR WHEN LEFT IN THE INCUBATOR FOR AN ADDITIONAL SIX DAYS WHAT WOULD THIS COLOR CHANGE INDICATE?. WHAT IS THE pH IN A NEGATIVE UREASE TEST?arrow_forwardWhich nutrient provides energy in its most concentrated form?arrow_forward
 Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning



