
Concept explainers
(a)
Interpretation:
The given item is chiral or achiral has to be identified.
Concept introduction:
Chiral:
The carbon atom which attached to the four different atoms is called as chiral carbon. A molecule is non superimposable on its mirror image is called chiral molecule. The molecule is called as chiral molecule and the carbon is called as chiral carbon.
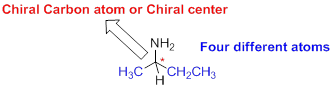
Achiral:
A molecule is superimposable on its mirror image is called achiral molecule.
(b)
Interpretation:
The given item is chiral or achiral has to be identified.
Concept introduction:
Chiral:
The carbon atom which attached to the four different atoms is called as chiral carbon. A molecule is non superimposable on its mirror image is called chiral molecule. The molecule is called as chiral molecule and the carbon is called as chiral carbon.

Achiral:
A molecule is superimposable on its mirror image is called achiral molecule.
(c)
Interpretation:
The given item is chiral or achiral has to be identified.
Concept introduction:
Chiral:
The carbon atom which attached to the four different atoms is called as chiral carbon. A molecule is non superimposable on its mirror image is called chiral molecule. The molecule is called as chiral molecule and the carbon is called as chiral carbon.

Achiral:
A molecule is superimposable on its mirror image is called achiral molecule.
(d)
Interpretation:
The given item is chiral or achiral has to be identified.
Concept introduction:
Chiral:
The carbon atom which attached to the four different atoms is called as chiral carbon. A molecule is non superimposable on its mirror image is called chiral molecule. The molecule is called as chiral molecule and the carbon is called as chiral carbon.

Achiral:
A molecule is superimposable on its mirror image is called achiral molecule.
Want to see the full answer?
Check out a sample textbook solution
Chapter 14 Solutions
Fundamentals Of General, Organic, And Biological Chemistry Volume 1 Second Custom Edition For Washington State University, 2/e
- Why is it not possible to find a five-fold symmetry operation in naturally-occurring crystals?arrow_forwardCellulose is a large polysaccharide containing many alpha(1→4) glycosidic linkages and alpha(1→6) glycosidic linkages. True or False?arrow_forwardWhich of the following structures exhibit geometric isomerism? Draw and name the two in each case.arrow_forward
- What properties do each of the following R groups have?arrow_forwardA compound with empirical formula C2H5O was found in a separate experiment to have a molar mass of approximately 90 g. What is the molecular formula of the compound?arrow_forwardDoes celluloid have something to do with cellulose?arrow_forward
- Following are two structural formulas for (S)-serine, one of the building blocks of proteins Is (S)-serine better represented by structural formula A or B?arrow_forwardWhat is chemically nonsensical about this structure? H-C=C-Harrow_forwardIn the 1970s, a process was developed thatconverts the glucose in corn syrup to its sweeter-tasting isomer, fructose.High-fructose corn syrup, a common ingredient in soft drinks and processedfood, is a mixture of glucose and fructose. What type of isomers are glucoseand fructose?arrow_forward
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON





