
(a)
Interpretation:
The major product obtained by the reaction of excess of given compound with bromine in presence of light at room temperature should be identified.
Concept introduction:
Radical or free radical: unpaired valence electron of an atom, molecule, or ion is called as radical.
Bond strength is depends on the formation of the radical, if the radical is involving in resonance which is weakest bond strength.
In a halogenation reaction, one or more halogen atoms are introduced into an organic compound. Generally, these reactions are initiated in the presence of light or heat.
Bromination:
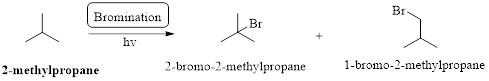
(b)
Interpretation:
The major product obtained by the reaction of excess of given compound with bromine in presence of light at room temperature should be identified.
Concept introduction:
Radical or free radical: unpaired valence electron of an atom, molecule, or ion is called as radical.
Bond strength is depends on the formation of the radical, if the radical is involving in resonance which is weakest bond strength.
In a halogenation reaction, one or more halogen atoms are introduced into an organic compound. Generally, these reactions are initiated in the presence of light or heat.
Bromination:
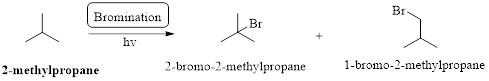
(c)
Interpretation:
The major product obtained by the reaction of excess of given compound with bromine in presence of light at room temperature should be identified.
Concept introduction:
Radical or free radical: unpaired valence electron of an atom, molecule, or ion is called as radical.
Bond strength is depends on the formation of the radical, if the radical is involving in resonance which is weakest bond strength.
In a halogenation reaction, one or more halogen atoms are introduced into an organic compound. Generally, these reactions are initiated in the presence of light or heat.
Bromination:
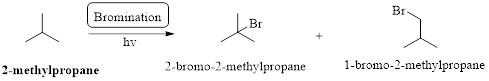
Want to see the full answer?
Check out a sample textbook solution
Chapter 14 Solutions
Essential Organic Chemistry (3rd Edition)
- For each of the following compounds, indicate all the products (ignoring stereochemistry) that could be formed when they undergo E2 reactions. If more than one E2 product is possible, indicate which one, if any, is the major product.arrow_forwardwhat is the major monoalkylation product you would expect to obtain of bromobenzene from reaction of chloromethane and AlCl3?arrow_forwardHow many distinct alkene products are possible when the alkyl iodide below undergoes E2 elimination?arrow_forward
- What would the major organic reaction product be from the reaction of 1-bromo-1-methylcyclopentane withsodium hydroxide? Would the elimination reaction outcome be affected if a student accidentally adds sodium tertbutoxide instead of sodium hydroxide?arrow_forwardName the products with any stereoisomers that can result when 2,2 dimethylbutane is subjected to free radical brominationarrow_forwardWhich of the following alkenes, upon reaction with Br2 in CCl4, produces a meso dibromo product?arrow_forward
- Conversion of an alkene to a halohydrin and internal displacement of a halide ion by an alkoxide ion are both stereoselective. Use this information to demonstrate that the configuration of the alkene is preserved in the epoxide. As an illustration, show that reaction of cis-2-butene by this two-step sequence gives cis-2,3- dimethyloxirane (cis-2-butene oxide).arrow_forwardSuppose you are told that each reaction is a substitution reaction but are not told the mechanism. Describe how you could conclude from the structure of the haloalkane, the nucleophile, and the solvent that each reaction is an SN2 reaction.arrow_forwardGive the product(s) that would be formed when 3-methyl-3-pentanol is subjected to acid-catalyzed dehydration. If more than one product would be formed, designate the one that would be the major product.arrow_forward
- When the compound shown below undergoes acid-catalyzed dehydration, a ring expansionoccurs to give the fused-ring product. What type of intermediate is formed in this reaction?Explain the ring expansion in terms of the reaction mechanismarrow_forwardComplete racemization had been observed in the product of a SN1 reaction of a haloalkane. How could thisexperimental observation be explained? Would you expect the same for SN2 reactions of haloalkanes?arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning


