
Interpretation:
The number of monochlorination products obtained from the radical chlorination of 2-methylbutane including all stereoisomers should be given.
Concept introduction:
Radical or free radical: unpaired valence electron of an atom, molecule, or ion is called as radical.
Bond strength is depends on the formation of the radical, if the radical is involving in resonance which is weakest bond strength.
In a halogenation reaction, one or more halogen atoms are introduced into an organic compound. Generally, these reactions are initiated in the presence of light or heat.
Chlorination:
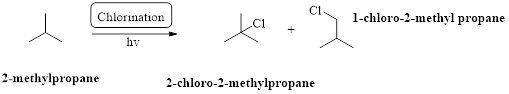
2-methylpropane undergoes radical chlorination which yields the 2-chloro-2-methylpropane and 1-chloro-2-methylpropane.
Chiral: Four different atoms attached to a carbon atom is called chiral molecule.
Stereoisomers: Stereoisomers are molecules that have the same molecular formula and they differ only in arrangement of atom in three-dimensional space.
Enantiomers: A compound which is non-superimposable mirror image is called enantiomers.
Diastereomers: A compound which is non-superimposable and non-mirror image is called enantiomers
Total number of stereoisomers = 2n
Where n is the number of chiral centers.
Want to see the full answer?
Check out a sample textbook solution
Chapter 14 Solutions
Essential Organic Chemistry (3rd Edition)
- Draw all stereoisomers formed by monochlorination of the cis andtrans isomers of 1,2-dimethylcyclobutane drawn below.arrow_forwardHow many stereoisomers of 2-methyl-heptan-4-ol could form? How many would you expect to form? In what ratios? (All equal?). Explain your answer Please explain clearly why the ratio is 1:1. Thank you!arrow_forwardOrg. Chem. 1. EA26. Can you please draw out all four , and explain what a stereoisomer is in relation ? Thanksarrow_forward
- Draw the products formed when both cis- and trans-but-2-ene are treated with OsO4, followed by hydrolysis with NaHSO3 + H2O. Explain how these reactions illustrate that syn dihydroxylation is stereospecific.arrow_forwardWhich group in the following pair is assigned the higher priority in R,Snomenclature −CH2NH2, −NHCH3arrow_forwardWhat are products formed from the below reaction?Draw the stereoisomers and name themarrow_forward
- What are products formed from the below reaction?Draw the stereoisomers and name them.(Hint: There are two different constitutional isomers and each constitutional isomer has 2nstereoisomers.arrow_forwardPlease identify priorities of functional groups and name following molecules based on thier stereochemistry as R or Sarrow_forwardReactions that target the substituents on arenes can change their behavior in EAS reactions.arrow_forward
- i. Fill in the missing starting materials, products, or reagents as necessary. ii. Label each transformation as SN1, SN2, or acid/base. iii. Indicate if the product is racemic or a single enantiomerarrow_forwardQ4 Which statement below about Sn1 reactions is incorrect? (A) SN1 reactions are stepwise and have intermediates. (B) The slow step in a SN1 reaction is formation of the carbocation intermediate. (C) SN1 reactions have first order kinetics which means only the alkyl halide is involved in the rate limiting step. (D) The products of a SN1 reaction will be a pair of enantiomers. (E) An aprotic solvent is best for Sn1 reactions as they tend to help stabilize carbocation intermediates.arrow_forwardWhat is the product (A, B, C, or D) of the reaction shown in Image30? a. C b.D c. B d. Aarrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
