
Chemistry & Chemical Reactivity
10th Edition
ISBN: 9781337399074
Author: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 9, Problem 53IL
The sulfamate ion, H2NSO3–, can be thought of as having been formed from the amide ion, NH2–, and sulphur trioxide, SO3.
(a) What are the electron-pair and molecular geometries or the amide ion and or SO3? What are the hybridizations of the N and S atoms, respectively?
(b) Sketch a structure for the sulfamate ion, and estimate the bond angles.
(c) What changes in hybridization do you expect for N and S in the course of the reaction
NH2– + SO3 → H2N—SO3–?
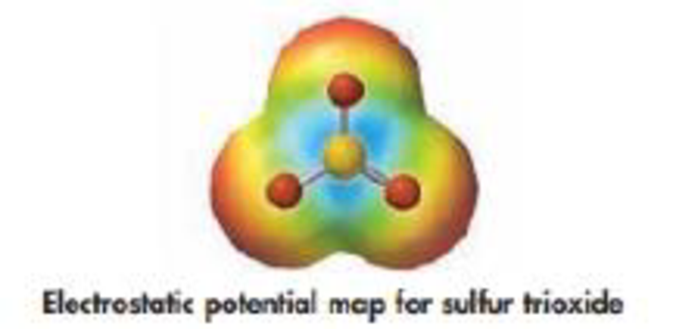
(d) Is SO3 the donor of an electron pair or the acceptor of an electron pair in the reaction with amide ion? Does the electrostatic potential map shown below confirm your prediction?
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
What atomic or hybrid orbitals make up the bond between C and O in carbon dioxide, CO2 ?
Complete the electron-dot structure of caffeine, showing all lone-pair electrons, and identify the hybridization of the indicated atoms.
What is the hybridization of the central atom in the sulfur trifluoride
SF−3 anion?
Chapter 9 Solutions
Chemistry & Chemical Reactivity
Ch. 9.1 - Use valence bond theory to describe the bonding in...Ch. 9.1 - Identify the hybridization of each underlined atom...Ch. 9.1 - Use valence bond theory to describe the bonding in...Ch. 9.2 - What is the electron configuration of the H2+ ion?...Ch. 9.2 - Could the anion Li2 exist? What is the ions bond...Ch. 9.2 - The cations O2+ and N2+ are formed when molecules...Ch. 9.3 - Photoelectron spectroscopy is s1milar to the...Ch. 9.3 - What is the energy of a photon with a wavelength...Ch. 9.3 - Using the accompanying figure, state which...Ch. 9.3 - The kinetic energy of an electron ejected from the...
Ch. 9.3 - The N2+ ions that are formed when electrons with...Ch. 9.3 - What is the empirical formula of Tynan purple?Ch. 9.3 - Butter yellow absorbs light with a wavelength of...Ch. 9.3 - Prob. 2.3ACPCh. 9 - Draw the Lewis structure for chloroform, CHCl3....Ch. 9 - Draw the Lewis structure for NF3. What are its...Ch. 9 - Draw the Lewis structure for hydroxylamine, H2NOH....Ch. 9 - Draw the Lewis structure for 1,...Ch. 9 - Draw the Lewis structure for carbonyl fluoride,...Ch. 9 - Draw the Lewis structure for acetamide, CH3CONH2....Ch. 9 - Specify the electron-pair and molecular geometry...Ch. 9 - Specify the electron-pair and molecular geometry...Ch. 9 - Prob. 9PSCh. 9 - What is the hybrid orbital set used by each of the...Ch. 9 - Draw the Lewis structures of the acid HPO2F2 and...Ch. 9 - Draw the Lewis structures of the arid HSO3F and...Ch. 9 - What is the hybridization of the carbon atom in...Ch. 9 - What is the hybridization of the carbon atoms in...Ch. 9 - What is the electron-pair and molecular geometry...Ch. 9 - What is the electron-pair and molecular geometry...Ch. 9 - Prob. 17PSCh. 9 - For each compound below, decide whether cis and...Ch. 9 - Molecular Orbital Theory (See Examples 9.49.6.)...Ch. 9 - Give the electron configurations for the ions Li2+...Ch. 9 - Calcium carbide, CaC2, contains the acetylide ion,...Ch. 9 - Platinum hexafluoride is an extremely strong...Ch. 9 - When sodium and oxygen react, one of the products...Ch. 9 - When potassium and oxygen react, one of the...Ch. 9 - Among the following, which has the shortest bond...Ch. 9 - Consider the following list of small molecules and...Ch. 9 - Prob. 27PSCh. 9 - The nitrosyl ion. NO+, has an interesting...Ch. 9 - These questions are not designated as to type or...Ch. 9 - What is the OSO angle and the hybrid orbital set...Ch. 9 - Sketch the resonance structures for the nitrite...Ch. 9 - Sketch the resonance structures for the nitrate...Ch. 9 - Sketch the resonance structures for the N2O...Ch. 9 - Compare the structure and bonding in CO2 and CO32...Ch. 9 - Numerous molecules are detected in deep space....Ch. 9 - Acrolein, a component of photochemical smog, has a...Ch. 9 - The organic compound below is a member of a class...Ch. 9 - The compound sketched below is acetylsalicylic...Ch. 9 - Phosphoserine is a less-common amino acid. (a)...Ch. 9 - Lactic acid is a natural compound found in sour...Ch. 9 - Cinnamaldehyde ocaus naturally in cinnamon oil....Ch. 9 - The ion Si2 was reported in a laboratory...Ch. 9 - The simple valence bond picture of O2 does not...Ch. 9 - Nitrogen, N2, can ionize to form N2+ or add an...Ch. 9 - Which of the homonuclear, diatomic molecules of...Ch. 9 - Which of the following molecules or ions are...Ch. 9 - Prob. 47GQCh. 9 - The structure of amphetamine, a stimulant, is...Ch. 9 - Menthol is used in soaps, perfumes, and foods. It...Ch. 9 - Prob. 50GQCh. 9 - Suppose you carry out the following reaction of...Ch. 9 - Ethylene oxide is an intermediate in the...Ch. 9 - The sulfamate ion, H2NSO3, can be thought of as...Ch. 9 - The compound whose structure is shown here is...Ch. 9 - Prob. 55ILCh. 9 - Carbon dioxide (CO2), dinitrogen monoxide (N2O),...Ch. 9 - Draw the two resonance structures that describe...Ch. 9 - Draw a Lewis structure for diimide, HNNH. Then,...Ch. 9 - Prob. 59SCQCh. 9 - Consider the three fluorides BF4, SiF4, and SF4....Ch. 9 - When two amino acids react with each other, they...Ch. 9 - What is the connection between bond order, bond...Ch. 9 - When is it desirable to use MO theory rather than...Ch. 9 - Show how valence bond theory and molecular orbital...Ch. 9 - Three of the four molecular orbitals for...Ch. 9 - Lets look more closely at the process of...Ch. 9 - Borax has the molecular formula Na2B4O5(OH)4. The...Ch. 9 - A model of the organic compound allene is shown...Ch. 9 - Prob. 69SCQCh. 9 - Prob. 70SCQCh. 9 - Bromine forms a number of oxides of varying...Ch. 9 - Prob. 72SCQCh. 9 - Urea reacts with malonic acid to produce...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Aspirin, or acetylsalicylic acid, has the formula C9H8O4 and the skeleton structure (a) Complete the Lewis structure and give the number of bonds and bonds in aspirin. (b) What is the hybridization about the CO2H carbon atom (colored blue)? (c) What is the hybridization about the carbon atom in the benzene-like ring that is bonded to an oxygen atom (colored red)? Also, what is the hybridization of the oxygen atom bonded to this carbon atom?arrow_forwardDescribe the hybridization around the central atom and the bonding in SCl2 and OCS.arrow_forwardConsider the polyatomic ion IO65-. How many pairs of electrons are around the central iodine atom? What is its hybridization? Describe the geometry of the ion.arrow_forward
- It is possible to write a simple Lewis structure for the SO42- ion, involving only single bonds, which follows the octet rule. However, Linus Pauling and others have suggested an alternative structure, involving double bonds, in which the sulfur atom is surrounded by six electron pairs. (a) Draw the two Lewis structures. (b) What geometries are predicted for the two structures? (c) What is the hybridization of sulfur in each case? (d) What are the formal charges of the atoms in the two structures?arrow_forwardAcrylonitrile, C3H3N is the building mer Orlon. Its Lewis structure is What is the hybridization of nitrogen and of the three numbered carbon atoms?arrow_forwardDescribe the bonding in the hydrogen cyanide molecule, HCN. Assume that N is sp-hybridized.arrow_forward
- What is the hybridization of the selenium atom in SeF4?arrow_forwardDescribe the bonding in pyridine, C5H5N, using hybrid orbitals.arrow_forward. Give the hybridization and oxidation state for sulfur in SO2, in SO3, and in H2SO4. And draw the Lewis structures to determine the hybridizationarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning
Stoichiometry - Chemistry for Massive Creatures: Crash Course Chemistry #6; Author: Crash Course;https://www.youtube.com/watch?v=UL1jmJaUkaQ;License: Standard YouTube License, CC-BY
Bonding (Ionic, Covalent & Metallic) - GCSE Chemistry; Author: Science Shorts;https://www.youtube.com/watch?v=p9MA6Od-zBA;License: Standard YouTube License, CC-BY
General Chemistry 1A. Lecture 12. Two Theories of Bonding.; Author: UCI Open;https://www.youtube.com/watch?v=dLTlL9Z1bh0;License: CC-BY