
Essential Organic Chemistry (3rd Edition)
3rd Edition
ISBN: 9780321937711
Author: Paula Yurkanis Bruice
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 14.6, Problem 10P
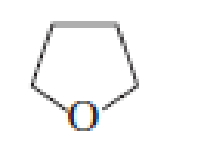
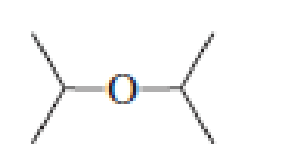
- a. Which ether is most apt to form a peroxide?
- b. Which ether is least apt to form a peroxide?
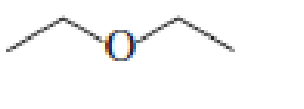
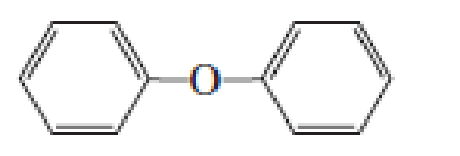
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Which ether is most apt to form a peroxide? Which ether is least apt to form a peroxide?
Explain why HF and HCl cannot be used to cleave ethers in an SN2 reaction.
Explain why an acetal can be isolated but most hydrates cannot be isolated.
Chapter 14 Solutions
Essential Organic Chemistry (3rd Edition)
Ch. 14.2 - Write the mechanism for the monobromination of...Ch. 14.2 - Prob. 2PCh. 14.4 - Prob. 3PCh. 14.4 - Which of the hydrogens in the structure in the...Ch. 14.4 - How many alkyl chlorides can be obtained from...Ch. 14.4 - Prob. 7PCh. 14.5 - Prob. 9PCh. 14.6 - a. Which ether is most apt to form a peroxide? b....Ch. 14.7 - Prob. 11PCh. 14 - Prob. 12P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- For the following reaction scheme, match the correct reagent to each reaction (A, B, C, D and E).arrow_forwardEthers are oxidized with O2 to form hydroperoxides that decompose violently when heated. Draw a stepwise mechanism for this reaction.arrow_forwardDraw a stepwise mechanism for the conversion of hex-5-en-1-ol to the cyclic ether A.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
Alcohols, Ethers, and Epoxides: Crash Course Organic Chemistry #24; Author: Crash Course;https://www.youtube.com/watch?v=j04zMFwDeDU;License: Standard YouTube License, CC-BY