
(a)
Interpretation:
The value of the rate constant for the decomposition of sulfuryl chloride at 600 K should be determined.
Concept Introduction:
Integrated rate laws for zero, first and second order reactions are,
Zeroth order:
First order:
Second order:
(a)
Answer to Problem 106AE
Explanation of Solution
If
| Time(hour) | 0.00 | 1.00 | 2.00 | 4.00 | 8.00 | 16.00 |
| Ptotal(atm) | 4.93 | 5.60 | 6.34 | 7.33 | 8.56 | 9.52 |
| PSO2Cl2 | 4.93 | 4.26 | 3.52 | 2.53 | 1.30 | 0.34 |
| Ln PSO2Cl2 | 1.595 | 1.449 | 1.258 | 0.928 | 0.262 | -1.08 |
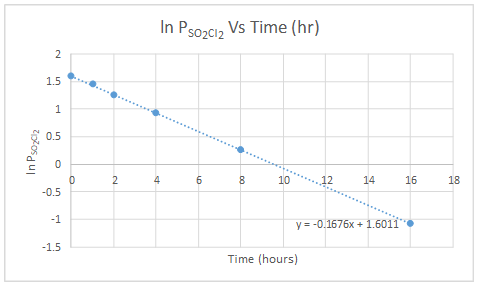
Pressure of gas is directly proportional to concentration.
The graph of ln PSO2Cl2 versus time is linear. So, the reaction of decomposition of SO2Cl2 is first order.
Integrated rate law for the reaction is,
The slope of the graph gives the value for rate constant.
(b)
Interpretation:
The half-life of the reaction should be calculated.
Concept Introduction:
Half-life for first order reaction can be calculated by,
(b)
Answer to Problem 106AE
Explanation of Solution
(c)
Interpretation:
The pressure in the vessel after 0.500 h and after 12.0 h should be calculated.
Concept Introduction:
Integrated rate law for the first order reaction;
[A]t − concentration of A at time t
[A]0 − initial concentration of A
k − rate constant
t − time
(c)
Answer to Problem 106AE
After 0.500 h = 5.33 atm
After 12.00 h = 9.20 atm
Explanation of Solution
After 0.500 h;
After 12.00 h;
(d)
Interpretation:
The fraction of the sulfuryl chloride remains after 20.0 h should be calculated.
Concept Introduction:
Integrated rate law for the first order reaction;
[A]t − concentration of A at time t
[A]0 − initial concentration of A
k − rate constant
t − time
(d)
Answer to Problem 106AE
Fraction of SO2Cl2 left =
Explanation of Solution
Fraction of SO2Cl2 left =
Want to see more full solutions like this?
Chapter 15 Solutions
Chemical Principles
- Hydrogen peroxide, H2O2(aq), decomposes to H2O() and O2(g) in a reaction that is first-order in H2O2 and has a rate constant k = 1.06 103 min1 at a given temperature. (a) How long will it take for 15% of a sample of H2O2 to decompose? (b) How long will it take for 85% of the sample to decompose?arrow_forwardAmmonia decomposes when heated according to the equation NH3(g) NH2(g) + H(g) The data in the table for this reaction were collected at a high temperature. Plot In [NH3] versus time and 1/[NH3] versus time. What is the order of this reaction with respect to NH3? Find the rate constant for the reaction from the slope.arrow_forwardSucrose, a sugar, decomposes in acid solution to give glucose and fructose. The reaction is first-order in sucrose, and the rate constant at 25 C is k = 0.21 h1. If the initial concentration of sucrose is 0.010 mol/L, what is its concentration after 5.0 h?arrow_forward
- Gaseous azomethane (CH3N2CH3) decomposes to ethane and nitrogen when heated: CH3N2CH3(g) CH3CH3(g) + N2(g) The decomposition of azomethane is a first-order reaction with k = 3.6 104 s1 at 600 K. (a) A sample of gaseous CH3N2CH3 is placed in a flask and heated at 600 K for 150 seconds. What fraction of the initial sample remains after this time? (b) How long must a sample be heated so that 99% of the sample has decomposed?arrow_forwardThe thermal decomposition of diacetylene, C4H2, was studied at 950 C. Use the following data (K. C. Hou and H. B. Palmer, Journal of Physical Chemistry. Vol. 60, p. 858, 1965) to determine the order of the reaction.arrow_forwardNitryl fluoride is an explosive compound that can be made by oxidizing nitrogen dioxide with fluorine: 2 NO2(g) + F2(g) → 2 NO2F(g) Several kinetics experiments, all done at the same temperature and involving formation of nitryl fluoride, are summarized in this table: Write the rate law for the reaction. Determine what the order of the reaction is with respect to each reactant and each product. Calculate the rate constant k and express it in appropriate units.arrow_forward
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781285199023Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781285199023Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning





