
(a)
Interpretation:
The Ptotal at
Concept Introduction:
Integrated rate laws for zero, first and second order reactions are,
Zeroth order:
First order:
Second order:
(a)
Answer to Problem 108AE
Explanation of Solution
The order of the reaction should be determined first.
Initial pressure of C2H5OH =
If x is the pressure of C2H5OH reacts, then at any time, t
| t (s) | PTotal (torr) | x (torr) | Pethanol (torr) |
| 0 | 250 | 0 | 250 |
| 10 | 265 | 15 | 235 |
| 20 | 280 | 30 | 220 |
| 30 | 295 | 45 | 205 |
| 40 | 310 | 60 | 190 |
| 50 | 325 | 75 | 175 |
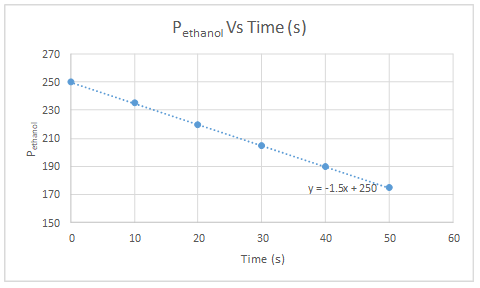
Pethanol versus time plot is linear. So, decomposition of ethanol follows zeroth order kinetics with respect to ethanol.
According to the equation on chart, Ptotal can be calculated as follows.
(b)
Interpretation:
The value of the rate constant and the units should be determined.
Concept Introduction:
Integrated rate laws for zero, first and second order reactions are,
Zeroth order:
First order:
Second order:
(b)
Answer to Problem 108AE
Explanation of Solution
Pethanol versus time plot is linear. So, decomposition of ethanol follows zeroth order kinetics with respect to ethanol.
Integrated rate law for the reaction;
So, the slope of the graph gives the value of the rate constant.
(c)
Interpretation:
The order of the reaction should be determined.
Concept Introduction:
Integrated rate laws for zero, first and second order reactions are,
Zeroth order:
First order:
Second order:
(c)
Answer to Problem 108AE
Zeroth order
Explanation of Solution
Pethanol versus time plot is linear. So, decomposition of ethanol follows zeroth order kinetics with respect to ethanol.
Integrated rate law for the reaction;
(d)
Interpretation:
Ptotal at
Concept Introduction:
Integrated rate laws for zero, first and second order reactions are,
Zeroth order:
First order:
Second order:
(d)
Answer to Problem 108AE
Explanation of Solution
According to the equation on chart, Ptotal can be calculated as follows.
But this is an impossible answer. Because the maximum pressure that can be after the completion of reaction is 500 torr.
When all the ethanol is decomposed
Therefore, at
Want to see more full solutions like this?
Chapter 15 Solutions
Chemical Principles
- The following rate constants were obtained in an experiment in which the decomposition of gaseous N2O; was studied as a function of temperature. The products were NO, and NO,. Temperature (K) 3.5 x 10_i 298 2.2 x 10"4 308 6.8 X IO-4 318 3.1 x 10 1 328 Determine Etfor this reaction in kj/mol.arrow_forwardThe frequency factor A is 6.31 108 L mol1 s1 and the activation energy is 10. kJ/mol for the gas-phase reaction NO(g)+O3(g)NO2(g)+O2(g) which is important in the chemistry of stratospheric ozone depletion. (a) Calculate the rate constant for this reaction at 370. K. (b) Assuming that this is an elementary reaction, calculate the rate of the reaction at 370. K if [NO] = 0.0010 M and [O3] = 0.00050 M.arrow_forwardDefine stability from both a kinetic and thermodynamic perspective. Give examples to show the differences in these concepts.arrow_forward
- The initial rate ( [NO]/ t] of the reaction of nitrogen monoxide and oxygen NO(g) + 2O2(g) NO2(g) was measured for various initial concentrations of NO and O2 at 25 C. Determine the rate equation from these data. What is the value of the rate constant, k, and what are its units?arrow_forwardHundreds of different reactions occur in the stratosphere, among them reactions that destroy the Earths ozone layer. The table below lists several (second-order) reactions of Cl atoms with ozone and organic compounds; each is given with its rate constant. For equal concentrations of Cl and the other reactant, which is the slowest reaction? Which is the fastest reaction?arrow_forwardThe decomposition of iodoethane in the gas phase proceeds according to the following equation: C2H5I(g)C2H4(g)+HI(g) At 660. K, k = 7.2 104 sl; at 720. K, k = 1.7 102 sl. What is the value of the rate constant for this first-order decomposition at 325C? If the initial pressure of iodoethane is 894 torr at 245C, what is the pressure of iodoethane after three half-lives?arrow_forward
- The decomposition of ozone is a second-order reaction with a rate constant of 30.6 atm1 s1 at 95 C. 2O3(g)3O2(g) If ozone is originally present at a partial pressure of 21 torr, calculate the length of time needed for the ozone pressure to decrease to 1.0 torr.arrow_forwardFor a reaction involving the decomposition of a hypothetical substance Y, these data are obtained: Determine the order of the reaction. Write the rate law for the decomposition of Y. Calculate k for the experiment above.arrow_forwardThe decomposition of N2O5 in CCl4 is a first-order reaction. If 2.56 mg of N2O5 is present initially and 2.50 mg is present after 4.26 minutes at 55 C, what is the value of the rate constant, k?arrow_forward
- Regular ?ights of supersonic aircraft in the stratosphere ale of concern because such aircraft produce nitric oxide, NO, as a byproduct in the exhaust of their engines. Nitric oxide reacts with ozone, and it has been suggested that this could contribute to depletion of the ozone layer. The reaction NO+O3NO2+O2 is first order with respect to both NO and O3 with a rate constant of 2.20107 L/mol/s. What is the instantaneous rate of disappearance of NO when [NO]=3.3106 M and [O3]=5.9107M?arrow_forwardWhen nitrogen dioxide reacts with carbon monoxide, the following reaction occurs. Â NO2(g)+CO(g)NO(g)+CO2(g)The following data are obtained at a certain temperature: (a) What is the order of the reaction with respect to NO2, CO, and overall? (b) Write the rate expression of the reaction. (c) Calculate k for the reaction. (d) When [ NO2 ]=0.421Mand [ CO ]=0.816M, what is the rate of the reaction at the temperature of the experiments?arrow_forwardMany biochemical reactions are catalyzed by acids. A typical mechanism consistent with the experimental results (in which HA is the acid and X is the reactant) is Step 1: Step 2: Step 3: Derive the rate law from this mechanism. Determine the order of reaction with respect to HA. Determine how doubling the concentration of HA would affect the rate of the reaction.arrow_forward
 Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781285199023Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781285199023Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning





