
(a)
Interpretation:
The average rate of decomposition of
Concept Introduction:
(a)
Answer to Problem 17E
The average rate of decomposition of
Explanation of Solution
The reaction is given as follows:
The given data is shown below:
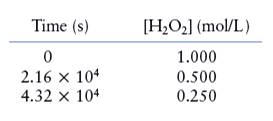
The rate of the reaction is given as follows:
The average rate of decomposition of
Therefore, the average rate of decomposition of
Similarly, the average rate of production of
Therefore, the average rate of production of
(b)
Interpretation:
The average rate of decomposition of
Concept Introduction:
Rate of reaction represents the change of concentration of a reactant or a product with respect to time. It can be expressed either by the reduce amount of reactant in per unit time or increase the amount of product in per unit time.
(b)
Answer to Problem 17E
The average rate of decomposition of
Explanation of Solution
The reaction is given as follows:
The given data is shown below:
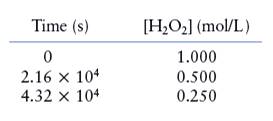
The rate of the reaction is given as follows:
The average rate of decomposition of
Therefore, the average rate of decomposition of
Similarly, the average rate of production of
Therefore, the average rate of production of
Want to see more full solutions like this?
Chapter 15 Solutions
Chemical Principles
- Ozone, O3, in the Earths upper atmosphere decomposes according to the equation 2 O3(g) 3 O2(g) The mechanism of the reaction is thought to proceed through an initial fast, reversible step followed by a slow, second step. Step 1: Fast, reversible O3(g) O2(g) + O(g) Step 2: Slow O3(g) + O(g) 2 O2(g) (a) Which of the steps is rate-determining? (b) Write the rate equation for the rate-determining steparrow_forwardAt 40C, H2O2 (aq) will decompose according to the following reaction: 2H2O2(aq)2H2O(l)+O2(g) The following data were collected for the concentration of H2O2 at various times. Times(s) [H2O2](mol/L) 0 1.000 2.16 104 0.500 4.32 104 0.250 a. Calculate the average rate of decomposition of H2O2 between 0 and 2.16 104 s. Use this rate to calculate the average rate of production of O2(g) over the same time period. b. What are these rates for the time period 2.16 104 s to 4.32 104 s?arrow_forwardThe following rate constants were obtained in an experiment in which the decomposition of gaseous N2O; was studied as a function of temperature. The products were NO, and NO,. Temperature (K) 3.5 x 10_i 298 2.2 x 10"4 308 6.8 X IO-4 318 3.1 x 10 1 328 Determine Etfor this reaction in kj/mol.arrow_forward
- The reaction 2 NO(g) + 2 H2(g) N2(g) + 2 H2O(g) was studied at 904 C, and the data in the table were collected. (a) Determine the order of the reaction for each reactant. (b) Write the rate equation for the reaction. (c) Calculate the rate constant for the reaction. (d) Find the rate of appearance of N2 at the instant when [NO] = 0.350 mol/L and [H] = 0.205 mol/L.arrow_forwardThe data in the table are for the reaction of NO and O2 at 660 K. NO(g) + O2(g) NO2(g) (a) Determine the order of the reaction for each reactant. (b) Write the rate equation for the reaction. (c) Calculate the rate constant. (d) Calculate the rate (in mol/L s) at the instant when [NO] = 0.015 mol/L and [O2] = 0.0050 mol/L. (e) At the instant when NO is reacting at the rate 1.0 104 mol/L s, what is the rate at which O2 is reacting and NO2 is forming?arrow_forwardFor each of the rate laws below, what is the order of the reaction with respect to the hypothetical substances X, Y, and Z? What is the overall order? (a) Rate = k [X][Y][Zl, (b) Rate = k [X]-[Y]1/2[Z], (c) Rate = k [X]L5[Y]-1, (d) Rate = k [X]/[Y]2arrow_forward
- Nitrogen monoxide reacts with hydrogen as follows: 2NO(g)+H2(g)N2O(g)+H2O(g) The rate law is [H2]/t = k[NO]2[H2], where k is 1.10 107 L2/(mol2 s) at 826C. A vessel contains NO and H2 at 826C. The partial pressures of NO and H2 are 144 mmHg and 315 mmHg, respectively. What is the rate of decrease of partial pressure of NO? See Problem 13.151.arrow_forwardThe reaction NO(g) + O,(g) — NO,(g) + 0(g) plays a role in the formation of nitrogen dioxide in automobile engines. Suppose that a series of experiments measured the rate of this reaction at 500 K and produced the following data; [NO] (mol L ’) [OJ (mol L 1) Rate = -A[NO]/Af (mol L_1 s-1) 0.002 0.005 8.0 X 10"'7 0.002 0.010 1.6 X 10-'6 0.006 0.005 2.4 X IO-'6 Derive a rate law for the reaction and determine the value of the rate constant.arrow_forwardAt 573 K, gaseous NO2(g) decomposes, forming NO(g) and O2(g). If a vessel containing NO2(g) has an initial concentration of 1.9 102 mol/L, how long will it take for 75% of the NO2(g) to decompose? The decomposition of NO2(g) is second-order in the reactant and the rate constant for this reaction, at 573 K, is 1.1 L/mol s.arrow_forward
 Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning





