
Interpretation:
The rate law should be calculated along with the value of rate constant for the given reaction.
Concept Introduction:
Rate law gives the relationship between the rate of the reaction and the reactant concentrations.
The general reaction is:
Rate of above reaction is expressed as:
Where, k = rate constant
The rate constant is defined as the proportionality constant which shows the relationship between concentration of reactants and rate of
Answer to Problem 27E
Rate law expression is:
The rate constant is equal to
Explanation of Solution
Given reaction:
The chemical reaction is:
The given data is:
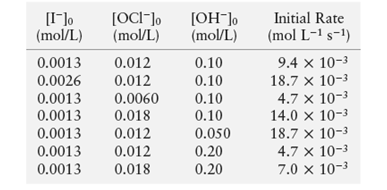
The given chemical reaction is:
The general rate law for above reaction is:
Here, concentration of hydroxide affect the rate, thus considered in rate law.
Where,
Now, put the values in above expression for first and second experiment.
Divide (1) and (2)
Hence, value of x = 1.
Now, put the values in above expression for first and third experiment.
Divide (1) and (3)
Hence, value of y = 1.
Now, put the values in above expression for first and fifth experiment.
Divide (1) and (4)
Hence, value of z=
Put the value of x, y and z in rate law expression:
The above expression is rearranged as:
Put the values in above expression from experiment 1 to determine the value of rate constant (k).
Thus, the rate constant is equal to
Want to see more full solutions like this?
Chapter 15 Solutions
Chemical Principles
- The Raschig reaction produces the industrially important reducing agent hydrazine, N2H4, from ammonia, NH3, and hypochlorite ion, OCl−, in basic aqueous solution. A proposed mechanism is Step 1: Step 2: Step 3: What is the overall stoichiometric equation? Which step is rate-limiting? What reaction intermediates are involved? What rate law is predicted by this mechanism?arrow_forwardWhen enzymes are present at very low concentration, their effect on reaction rate can be described by first-order kinetics. Calculate by what factor the rate of an enzyme-catalyzed reaction changes when the enzyme concentration is changed from 1.5 107 M to 4.5 106 M.arrow_forwardMany biochemical reactions are catalyzed by acids. A typical mechanism consistent with the experimental results (in which HA is the acid and X is the reactant) is Step 1: Step 2: Step 3: Derive the rate law from this mechanism. Determine the order of reaction with respect to HA. Determine how doubling the concentration of HA would affect the rate of the reaction.arrow_forward
- At 573 K, gaseous NO2(g) decomposes, forming NO(g) and O2(g). If a vessel containing NO2(g) has an initial concentration of 1.9 102 mol/L, how long will it take for 75% of the NO2(g) to decompose? The decomposition of NO2(g) is second-order in the reactant and the rate constant for this reaction, at 573 K, is 1.1 L/mol s.arrow_forwardSucrose, a sugar, decomposes in acid solution to give glucose and fructose. The reaction is first-order in sucrose, and the rate constant at 25 C is k = 0.21 h1. If the initial concentration of sucrose is 0.010 mol/L, what is its concentration after 5.0 h?arrow_forwardNitramide, NO2NH2, decomposes slowly in aqueous solution according to the following reaction: NO2NH2(aq) N2O(g) + H2O() The reaction follows the experimental rate law Rate=k[NO2NH2][H3O+] (a) What is the apparent order of the reaction in a pH buffered solution? (In a pH buffered solution, the concentration of H3O+ is a constant.) (b) Which of the following mechanisms is the most appropriate for the interpretation of this rate law? Explain. (Note that when writing the expression for K, the equilibrium constant, [H2O] is not involved. See Chapter 15.) Mechanism 1 NO2NH2K1N2O+H2O Mechanism 2 NO2NH2+H3O+k2k2NO2NH3++H2O(rapidequilibrium) NO2NH3+k3N2O+H3O+(rate-limitingstep) Mechanism 3 NO2NH2+H2Ok4k4NO2NH+H3O+(rapidequilibrium)NO2NHk5N2O+OH(rate-limitingstep)H3O++OHk62H2O(veryfastreaction) (c) Show the relationship between the experimentally observed rate constant, k, and the rate constants in the selected mechanism. (d) Based on the experimental rate law, will the reaction rate increase or decrease if the pH of the solution is increased?arrow_forward
- For the reaction of phenyl acetate with water the concentration as a function of time was given in Question 11. Assume that the concentration of water does not change during the reaction. Analyze the data from Question 11 to determine (a) the rate law. (b) the order of the reaction with respect to phenyl acetate. (c) the rate constant. (d) the rate of reaction when the concentration of phenyl acetate is 0.10 mol/L (assuming that the concentration of water is the same as in the experiments in the table in Question 11).arrow_forwardUnder certain conditions the decomposition of ammonia on a metal surface gives the following data: [NH3] (M) 1.0103 2.0103 3.0103 Rate (moI/L/h1) 1.5106 1.5106 1.5106 Determine the rate equation, the rate constant, and the overall order for this reaction.arrow_forwardExplain why half-lives are not normally used to describe reactions other than first order.arrow_forward
- The decomposition of many substances on the surface of a heterogeneous catalyst shows the following behavior: How do you account for the rate law changing from first order to zero order in the concentration of reactant?arrow_forwardFor the reaction of iodine atoms with hydrogen molecules in the gas phase, these rate constants were obtained experimentally. 2I(g) + H2(g) 2HI(g) (a) Calculate the activation energy and frequency factor for this reaction. (b) Estimate the rate constant of the reaction at 400.0 K.arrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStaxChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStaxChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning





