
Concept explainers
(a)
Interpretation:
An anode in the given reaction should be determined.
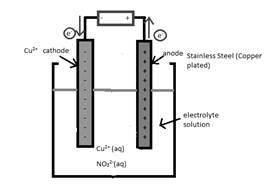
Concept Introduction:
Electrolytic cell uses an
The reactant which loses electrons during the reaction is oxidized whereas the reactant which gains electrons during the reaction is reduced.
An electrode where oxidation that is increase in oxidation number takes place is known as anode.
An electrode where reduction that is decrease in oxidation number takes place is known as cathode.
A reaction which is either oxidation or reduction reaction component of redox reaction is known as half-reaction. It is obtained by considering the oxidation states changes of the reactant and product in the redox reaction.
(b)
Interpretation:
A cathode in the given reaction should be determined.
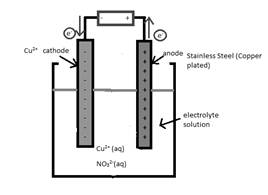
Concept Introduction:
Electrolytic cell uses an electric current to derive a nonspontaneous oxidation-reduction reaction and process in known as electrolysis. In oxidation-reduction reaction, the transfer of electrons occurs from one reactant to another reactant.
The reactant which loses electrons during the reaction is oxidized whereas the reactant which gains electrons during the reaction is reduced.
An electrode where oxidation that is increase in oxidation number takes place is known as anode.
An electrode where reduction that is decrease in oxidation number takes place is known as cathode.
A reaction which is either oxidation or reduction reaction component of redox reaction is known as half-reaction. It is obtained by considering the oxidation states changes of the reactant and product in the redox reaction.
(c)
Interpretation:
The half-reaction which occurs at the anode should be written.
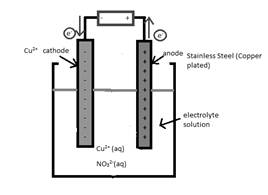
Concept Introduction:
Electrolytic cell uses an electric current to derive a nonspontaneous oxidation-reduction reaction and process in known as electrolysis. In oxidation-reduction reaction, the transfer of electrons occurs from one reactant to another reactant.
The reactant which loses electrons during the reaction is oxidized whereas the reactant which gains electrons during the reaction is reduced.
An electrode where oxidation that is increase in oxidation number takes place is known as anode.
An electrode where reduction that is decrease in oxidation number takes place is known as cathode.
A reaction which is either oxidation or reduction reaction component of redox reaction is known as half-reaction. It is obtained by considering the oxidation states changes of the reactant and product in the redox reaction.
(d)
Interpretation:
The half-reaction which occurs at the cathode should be written.
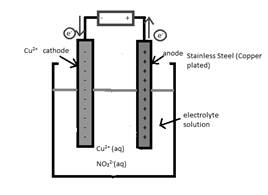
Concept Introduction:
Electrolytic cell uses an electric current to derive a nonspontaneous oxidation-reduction reaction and process in known as electrolysis. In oxidation-reduction reaction, the transfer of electrons occurs from one reactant to another reactant.
The reactant which loses electrons during the reaction is oxidized whereas the reactant which gains electrons during the reaction is reduced.
An electrode where oxidation that is increase in oxidation number takes place is known as anode.
An electrode where reduction that is decrease in oxidation number takes place is known as cathode.
A reaction which is either oxidation or reduction reaction component of redox reaction is known as half-reaction. It is obtained by considering the oxidation states changes of the reactant and product in the redox reaction.
Want to see the full answer?
Check out a sample textbook solution
Chapter 15 Solutions
Basic Chemistry (5th Edition)
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





