
(a)
Interpretation:
The structural formula of missing compound in the given reaction has to be drawn.
Concept Introduction:
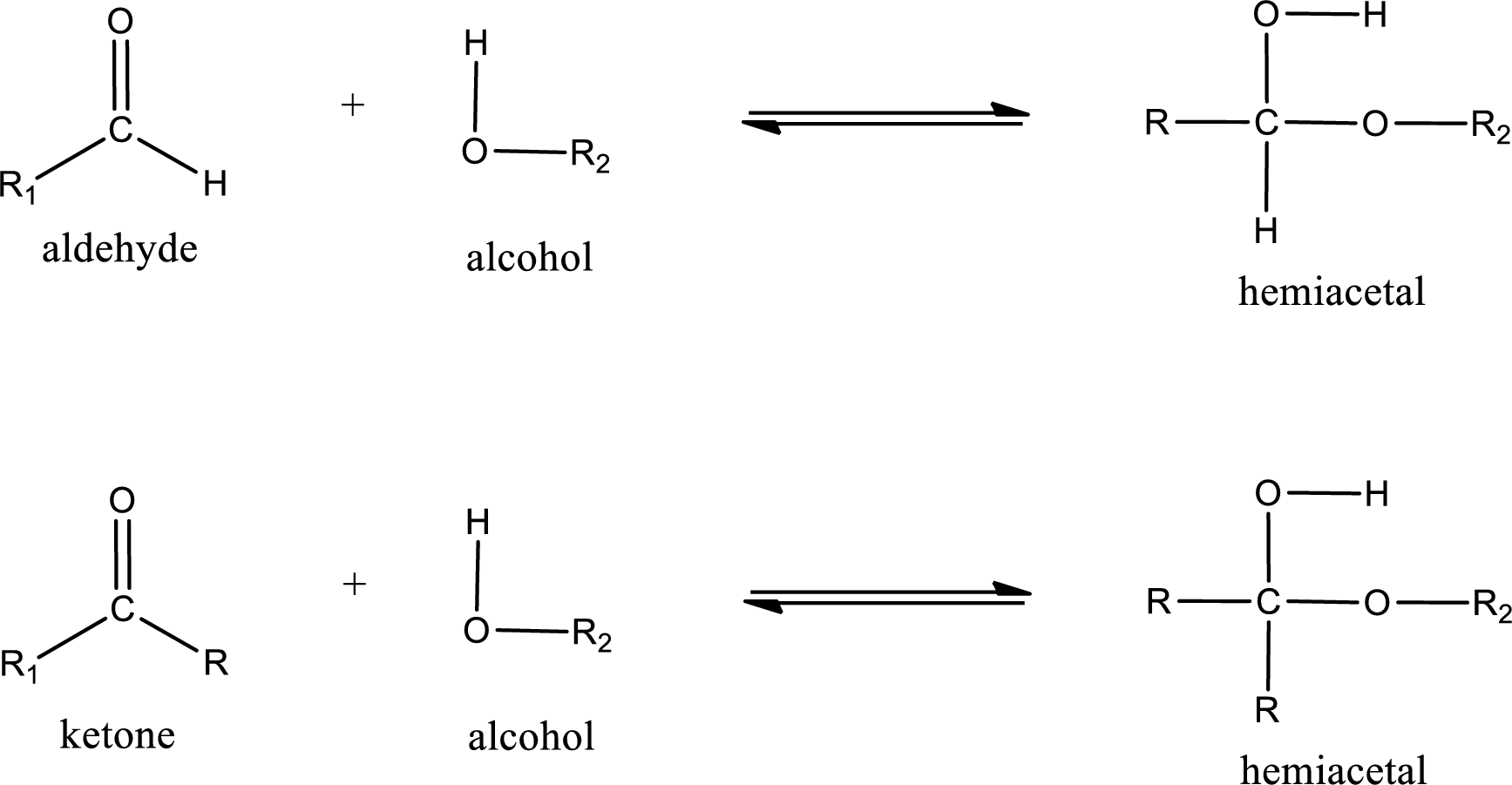
Aldehydes and ketones react with alcohol to form hemiacetal as the product. This reacts with further molecule of aldehyde or ketone to form acetal.
Hemiacetal is an addition product that is obtained by reaction between aldehyde or ketone with alcohol. The general reaction of hemiacetal formation can be given as,
(b)
Interpretation:
The structural formula of missing compound in the given reaction has to be drawn.
Concept Introduction:
Aldehydes contain a carbonyl group that is bonded to a hydrogen atom and a carbon atom. Ketones are compounds that contain a carbonyl group bonded to two carbon atoms. Aldehydes and ketones undergo addition reaction across the carbonyl group.
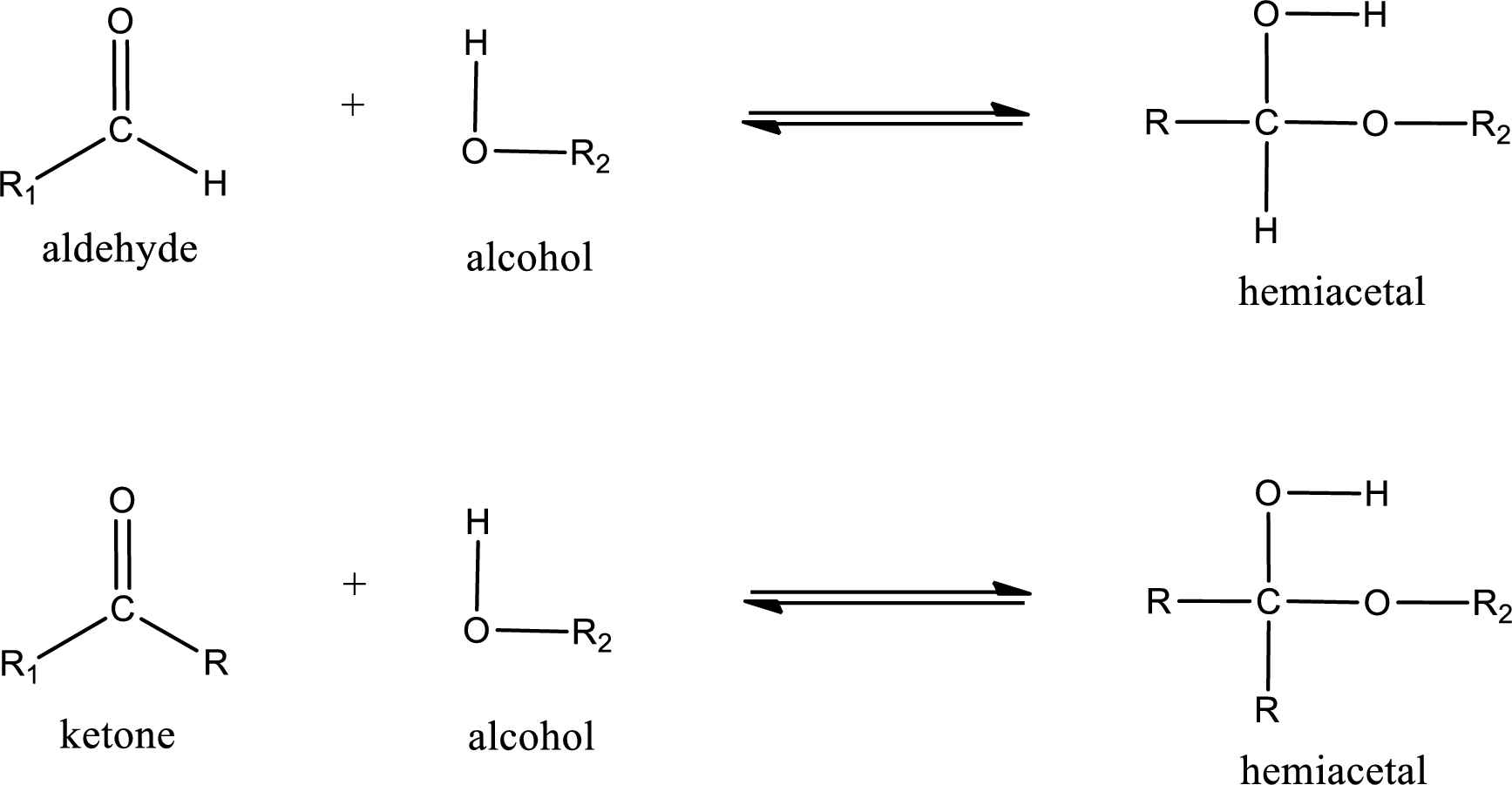
Aldehydes and ketones react with alcohol to form hemiacetal as the product. This reacts with further molecule of aldehyde or ketone to form acetal.
Hemiacetal is an addition product that is obtained by reaction between aldehyde or ketone with alcohol. The general reaction of hemiacetal formation can be given as,
(c)
Interpretation:
The structural formula of missing compound in the given reaction has to be drawn.
Concept Introduction:
Aldehydes contain a carbonyl group that is bonded to a hydrogen atom and a carbon atom. Ketones are compounds that contain a carbonyl group bonded to two carbon atoms. Aldehydes and ketones undergo addition reaction across the carbonyl group.
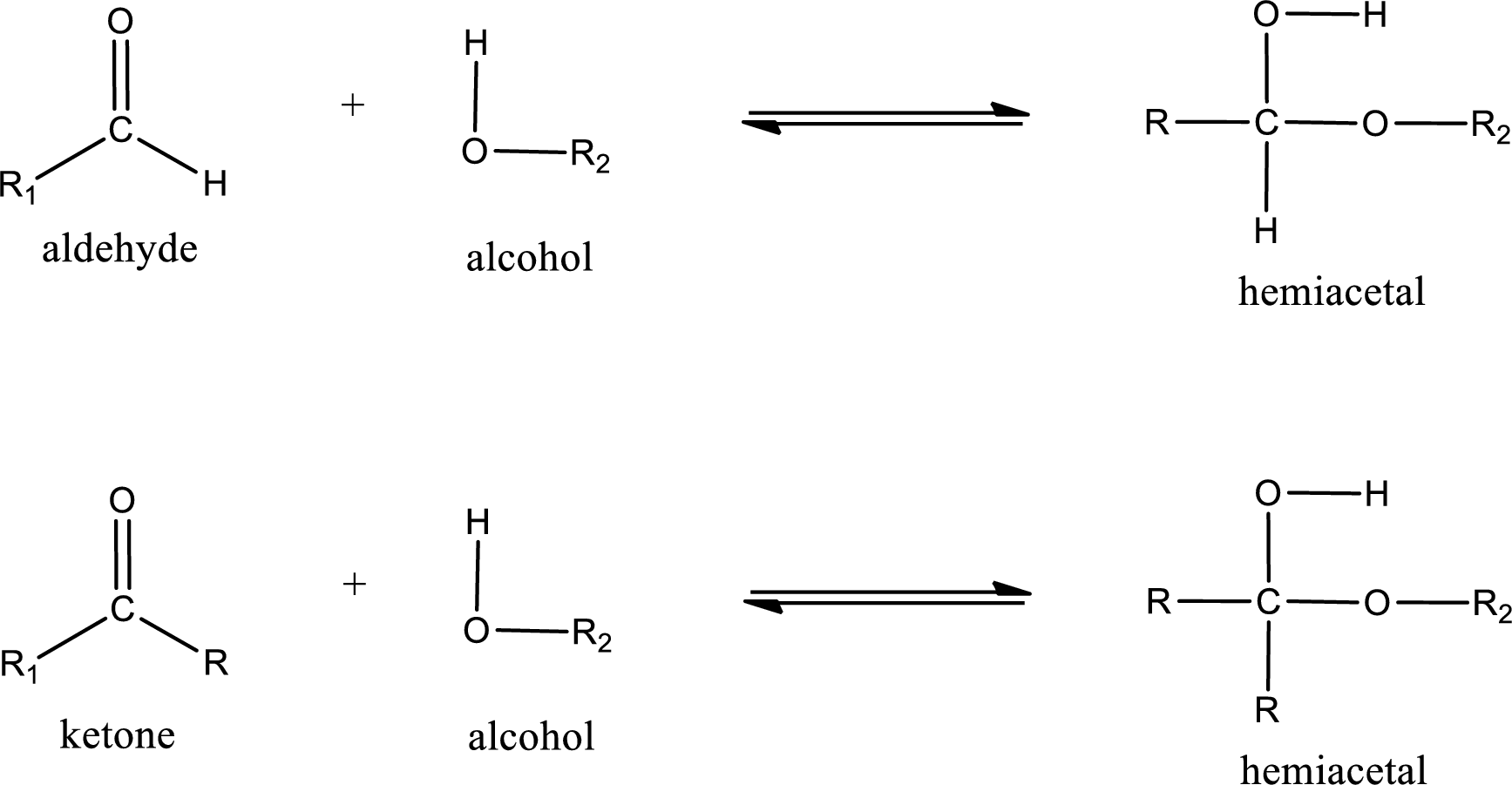
Aldehydes and ketones react with alcohol to form hemiacetal as the product. This reacts with further molecule of aldehyde or ketone to form acetal.
Hemiacetal is an addition product that is obtained by reaction between aldehyde or ketone with alcohol. The general reaction of hemiacetal formation can be given as,
(d)
Interpretation:
The structural formula of missing compound in the given reaction has to be drawn.
Concept Introduction:
Aldehydes contain a carbonyl group that is bonded to a hydrogen atom and a carbon atom. Ketones are compounds that contain a carbonyl group bonded to two carbon atoms. Aldehydes and ketones undergo addition reaction across the carbonyl group.
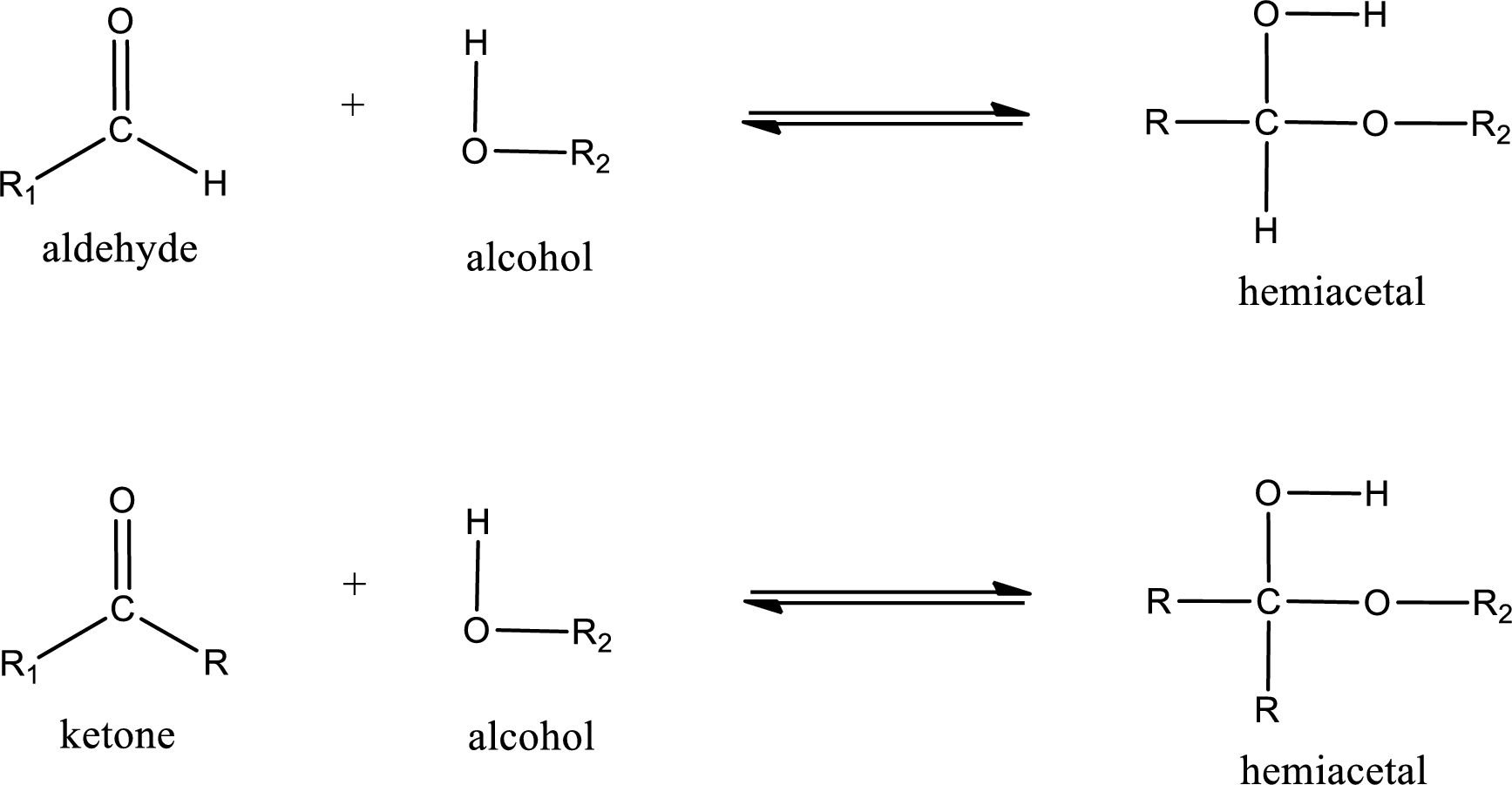
Aldehydes and ketones react with alcohol to form hemiacetal as the product. This reacts with further molecule of aldehyde or ketone to form acetal.
Hemiacetal is an addition product that is obtained by reaction between aldehyde or ketone with alcohol. The general reaction of hemiacetal formation can be given as,
Trending nowThis is a popular solution!

Chapter 15 Solutions
Bundle: General, Organic, and Biological Chemistry, 7th + OWLv2 Quick Prep for General Chemistry, 4 terms (24 months) Printed Access Card
- Fumaric acid is a metabolic intermediate that has the systematic name trans-2-butenedioic acid. Draw its structure.arrow_forward2-butanol can be formed as the only product of the Markovnikov addition of H2O to two different alkenes. In contrast, 2-octanol can be formed as the only product of the Markovnikov addition of H2O to just one alkene. To examine the difference, draw the alkene starting materials of each alcohol. Draw the bond-line (skeletal) structures of the two alkene starting materials that can be used to synthesize 2-butanol via Markovnikov hydration. Part 1 of 2 Click and drag to start drawing a structure. : ☐ ☑ ⑤arrow_forwardDraw the structures of the following acids:(a) 2-Ethyl-3-hydroxyhexanoic acid (b) m-Nitrobenzoic acidarrow_forward
- Draw Lewis structures for two compounds of formula C2H7N.arrow_forwardSalicylic acid (o-hydroxybenzoic acid) is used as starting material to prepare aspirin. Draw the structure of salicylic acid.arrow_forwardDraw the skeletal structure of the alkene that is needed as a starting material to prepare each of the following alcohols. Part 1 of 2 CH3CH(OH)CH3 Click and drag to start drawing a structure. ☑ :arrow_forward
- Draw all possible carboxylic acids with the formula C5H10O2.arrow_forwardFor the following reactions, identify the atom(s) being oxidized and reduced:arrow_forwardDraw a structure for the compound, C3H5Br, that fits the following 1H NMR data: δ 2.32 (3H, singlet) δ 5.35 (1H, broad singlet) δ 5.54 (1H, broad singlet)arrow_forward
- Drawn are four isomeric dimethylcyclopropanes. How are the compounds in each pair related (enantiomers, diastereomers, constitutional isomers): A and B; A and C; B and C; C and D?arrow_forwardIn the preparation of aspirin, You can do the functional group test to ensure the completion of the reaction. (a) What is the name of reagent used? (b) What is your observation if any unreacted starting material is present? (c) What is the name of the functional group responsible for this reaction?arrow_forwardIf the phosphorus atom in 3-phosphoglycerate is radioactively labeled, where will the label be when the reaction that forms 2-phosphoglycerate is over?arrow_forward
 Human Anatomy & Physiology (11th Edition)BiologyISBN:9780134580999Author:Elaine N. Marieb, Katja N. HoehnPublisher:PEARSON
Human Anatomy & Physiology (11th Edition)BiologyISBN:9780134580999Author:Elaine N. Marieb, Katja N. HoehnPublisher:PEARSON Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax
Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax Anatomy & PhysiologyBiologyISBN:9781259398629Author:McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa StouterPublisher:Mcgraw Hill Education,
Anatomy & PhysiologyBiologyISBN:9781259398629Author:McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa StouterPublisher:Mcgraw Hill Education, Molecular Biology of the Cell (Sixth Edition)BiologyISBN:9780815344322Author:Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter WalterPublisher:W. W. Norton & Company
Molecular Biology of the Cell (Sixth Edition)BiologyISBN:9780815344322Author:Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter WalterPublisher:W. W. Norton & Company Laboratory Manual For Human Anatomy & PhysiologyBiologyISBN:9781260159363Author:Martin, Terry R., Prentice-craver, CynthiaPublisher:McGraw-Hill Publishing Co.
Laboratory Manual For Human Anatomy & PhysiologyBiologyISBN:9781260159363Author:Martin, Terry R., Prentice-craver, CynthiaPublisher:McGraw-Hill Publishing Co. Inquiry Into Life (16th Edition)BiologyISBN:9781260231700Author:Sylvia S. Mader, Michael WindelspechtPublisher:McGraw Hill Education
Inquiry Into Life (16th Edition)BiologyISBN:9781260231700Author:Sylvia S. Mader, Michael WindelspechtPublisher:McGraw Hill Education





