
(a)
Interpretation:
The splitting pattern is to be determined for the type of H highlighted in the given molecule.
Concept introduction:
Each chemically distinct proton generates a signal in proton nmr spectroscopy. The signal may be modified, split into a number of lines, by nearby protons. Typically only protons three bonds away produce distinct splitting of the signal of interest. The number of lines, called multiplicity, into which the signal is split, equals
Answer to Problem 16.58P
The signal for the highlighted H will be a singlet.
Explanation of Solution
The structure of the molecule with the specific H highlighted is
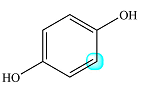
The signal for that H can be split by any protons on adjacent carbons.
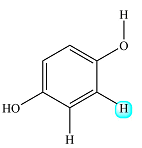
There is only one such proton. The proton on the nearest OH group is four bonds away, so it will not have any effect. Only the proton on the left is close enough to have an effect, but it is structurally equivalent to the one of interest. Structurally identical protons do not cause splitting of a signal.
Therefore, the signal of the highlighted proton is not split and appears as a singlet.
The multiplicity of the signal is determined on the basis of the number of structurally distinct protons coupled to it.
(b)
Interpretation:
The splitting pattern is to be determined for the type of H highlighted in the given molecule.
Concept introduction:
Each chemically distinct proton generates a signal in proton nmr spectroscopy. The signal may be modified, split into a number of lines, by nearby protons. Typically only protons three bonds away produce distinct splitting of the signal of interest. The number of lines, called multiplicity, into which the signal is split, equals
The intensities of the individual lines in such signals are in fixed ratios, given by Pascal’s triangle.

Answer to Problem 16.58P
The signal for the highlighted proton will appear to be a multiplet, with nine peaks.
Explanation of Solution
The structure of the molecule with the highlighted proton is
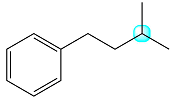
The highlighted proton has a total of eight coupled protons, six from the two methyl groups and two from the methylene bridge.
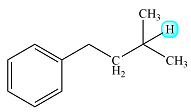
Although the methyl protons and the methylene protons are structurally not exactly equivalent, their coupling constants are similar. Therefore, instead of the complex splitting expected of two distinctly differernt proton groups, they will cause a splitting that resembles the splitting by eight equivalent protons. Therefore, the signal will be split into a multiplet with nine peaks.
The multiplicity of the signal is determined on the basis of the number of structurally distinct protons coupled to it.
(c)
Interpretation:
The splitting pattern is to be determined for the type of H highlighted in the given molecule.
Concept introduction:
Each chemically distinct proton generates a signal in proton nmr spectroscopy. The signal may be modified, split into a number of lines, by nearby protons. Typically only protons three bonds away produce distinct splitting of the signal of interest. The number of lines, called multiplicity, into which the signal is split, equals
Answer to Problem 16.58P
The signal of the first highlighted protons, from the end methyl group will be split into a triplet. The signal for the second highlighted type will be split into a quartet.
Explanation of Solution
The molecule and the highlighted protons are
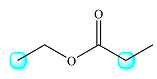
The protons coupled to each of these are shown in the drawing below:
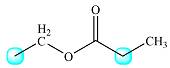
The one on the left is coupled to two protons from the methylene group. Therefore, the signal for the first type of protons will be split into a triplet.
The second group is coupled to three protons from the methyl group to its right. Therefore, this signal will be split into a quartet.
The multiplicity of the signal is determined on the basis of the number of structurally distinct protons coupled to it.
(d)
Interpretation:
The splitting pattern is to be determined for the type of H highlighted in the given molecule.
Concept introduction:
Each chemically distinct proton generates a signal in proton nmr spectroscopy. The signal may be modified, split into a number of lines, by nearby protons. Typically only protons three bonds away produce distinct splitting of the signal of interest. The number of lines, called multiplicity, into which the signal is split, equals
Answer to Problem 16.58P
The signal for the given proton is split into a doublet.
Explanation of Solution
The structure of the molecule with the highlighted proton is
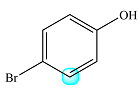
Only one proton is coupled to the highlighted one. Both are
Therefore, the signal of the highlighted proton will be split into a doublet.
The multiplicity of the signal is determined on the basis of the number of structurally distinct protons coupled to it.
(e)
Interpretation:
The splitting pattern is to be determined for the type of H highlighted in the given molecule.
Concept introduction:
Each chemically distinct proton generates a signal in proton nmr spectroscopy. The signal may be modified, split into a number of lines, by nearby protons. Typically only protons three bonds away produce distinct splitting of the signal of interest. The number of lines, called multiplicity, into which the signal is split, equals
Answer to Problem 16.58P
The signal of the first highlighted H is a triplet and that of the second is a singlet.
Explanation of Solution
The structure of the molecule with the highlighted protons is
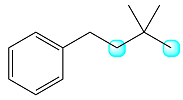
The first of these, in the middle of the chain, has two coupled protons while the second, at the end, has no coupled protons.
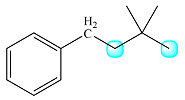
Therefore, the signal of the first one will be split into a triplet while that of the second will remain a singlet.
The multiplicity of the signal is determined on the basis of the number of structurally distinct protons coupled to it.
(f)
Interpretation:
The splitting pattern is to be determined for the type of H highlighted in the given molecule.
Concept introduction:
Each chemically distinct proton generates a signal in proton nmr spectroscopy. The signal may be modified, split into a number of lines, by nearby protons. Typically only protons three bonds away produce distinct splitting of the signal of interest. The number of lines, called multiplicity, into which the signal is split, equals
Answer to Problem 16.58P
The splitting pattern for the given H will be a doublet of triplets (or a triplet of doublets).
Explanation of Solution
The structure of the molecule with the highlighted H is
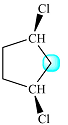
There are two protons present at the indicated position. They appear to be structurally equivalent, but they are actually diastereotopic. Each one of them is coupled to two identical protons (Hc), as shown below:
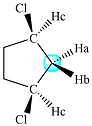
So signal for each proton (Ha and Hb) is split into a triplet by the two coupled protons (Hc). So effectively, the signal will be a doublet of triplets.
The multiplicity of the signal is determined on the basis of the number of structurally distinct protons coupled to it.
Want to see more full solutions like this?
Chapter 16 Solutions
ORGANIC CHEMISTRY E-BOOK W/SMARTWORK5
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





