
(a)
Interpretation:
The product (organic ion) formed in the given reaction should be named.
Concept introduction:
Depending on the number of carbon side chain of the nitrogen, different types of amines can form.
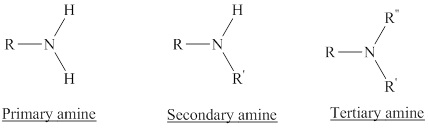
In a quaternary ammonium ion a nitrogen atom with four attached groups is positively charged. And their compounds are known as quaternary ammonium salt.
Reactions of these types of amine with
Primary amines can be named in the IUPAC system in several ways,
For simple amines the suffix – amine is added to the name of the alkyl substituent.
The suffix-amine can be used in place of the final –e in the name of the parent compound.
For a secondary amine an N prefixes the compound giving the shorter carbon chain and its chain prefix name.
For a tertiary amine an N, N prefixes the compound giving the two shorter carbon chains and their side chain prefix names.
(b)
Interpretation:
The product (organic ion) formed in the given reaction should be named.
Concept introduction:
Amines are the derivatives of ammonia
Depending on the number of carbon side chain of the nitrogen, different types of amines can form.

In a quaternary ammonium ion a nitrogen atom with four attached groups is positively charged. And their compounds are known as quaternary ammonium salt.
Reactions of these types of amine with
Primary amines can be named in the IUPAC system in several ways,
For simple amines the suffix – amine is added to the name of the alkyl substituent.
The suffix-amine can be used in place of the final –e in the name of the parent compound.
For a secondary amine an N prefixes the compound giving the shorter carbon chain and its chain prefix name.
For a tertiary amine an N, N prefixes the compound giving the two shorter carbon chains and their side chain prefix names.
Aniline is an
(c)
Interpretation:
The product formed in the given reaction should be named.
Concept introduction:
Amines are the derivatives of ammonia
Depending on the number of carbon side chain of the nitrogen, different types of amines can form.

In a quaternary ammonium ion a nitrogen atom with four attached groups is positively charged. And their compounds are known as quaternary ammonium salt.
Reactions of these types of amine with
Primary amines can be named in the IUPAC system in several ways,
For simple amines the suffix – amine is added to the name of the alkyl substituent.
The suffix-amine can be used in place of the final –e in the name of the parent compound.
For a secondary amine an N prefixes the compound giving the shorter carbon chain and its chain prefix name.
For a tertiary amine an N, N prefixes the compound giving the two shorter carbon chains and their side chain prefix names.
Want to see the full answer?
Check out a sample textbook solution
Chapter 16 Solutions
Fund. of General, Org... -Masteringchem.
- Ammonia, NH3, and phosphorus trihydride, PH3, both have trigonal pyramid geometry. Which one is more polar? Explain.arrow_forward1-34. Indicate whether each of the following statements about enantiomers is true or false. (a to d)arrow_forwardPropanamide and methyl acetate have about the same molar mass, both are quite soluble in water, and yet the boiling point of propanamide is 486 K, whereas that of methyl acetate is 330 K. Explain.arrow_forward
- The liquids butan-1-ol and butanal have similar molar masses. Which is expected to have the higher boiling point? Explain your choices.arrow_forwardIf the dehydration reaction of an alcohol is successful, what changes would be seen in the IR spectrum for the product compared to the starting materialarrow_forwardIdentify the acid on the left and its conjugate base on the right in the following equations:arrow_forward
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON





