
(a)
Interpretation:
For the given reaction, the major product and reaction mechanism is to be drawn.
Concept introduction:
The weak resonance contributor with separated charges generates a small partial positive charge on the beta carbon in the resonance hybrid. Thus, a nucleophile can attack at either the carbonyl carbon or the beta carbon. A polar
Answer to Problem 17.60P
The nucleophile adds reversibly and undergoes a 1, 4-addition reaction.
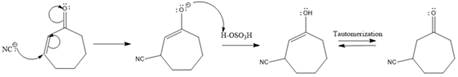
Explanation of Solution
The weak resonance contributor with separated charges generates a small partial positive charge on the beta carbon in the resonance hybrid. Thus, a nucleophile can attack at either the carbonyl carbon or the beta carbon. A polar
In this reaction, both carbonyl carbon as well as beta carbon are electrophilic. Here the nucleophile adds reversibly; therefore, this reaction undergoes 1, 4-addition. In this reaction,
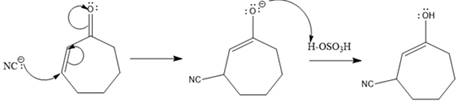
Here the enol tautomerizes back to the
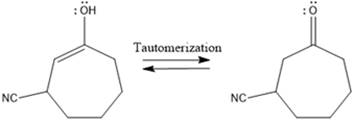
From the location of the electrophilic carbon, the major product for the given reaction is predicted, and the reaction mechanism is drawn.
(b)
Interpretation:
For the given reaction, the major product and the reaction mechanism is to be drawn.
Concept introduction:
The weak resonance contributor with separated charges generates a small partial positive charge on the beta carbon in the resonance hybrid. Thus, a nucleophile can attack at either the carbonyl carbon or the beta carbon. A polar
Answer to Problem 17.60P
The nucleophile adds irreversibly and undergoes a 1, 2-addition reaction.
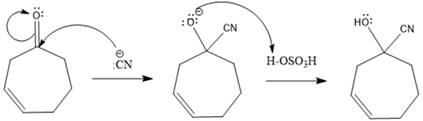
Explanation of Solution
A polar
In this reaction, the nucleophile adds irreversibly; hence this reaction undergoes direction addition (1, 2-addition). In this reaction,
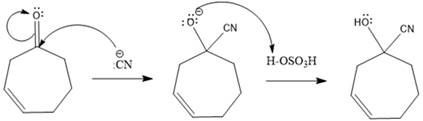
From the location of the electrophilic carbon, the major product for the given reaction is predicted, and the reaction mechanism is drawn.
(c)
Interpretation:
For the given reaction, the major product and the reaction mechanism is to be drawn.
Concept introduction:
The weak resonance contributor with separated charges generates a small partial positive charge on the beta carbon in the resonance hybrid. Thus, a nucleophile can attack at either the carbonyl carbon or the beta carbon. A polar
Answer to Problem 17.60P
The nucleophile adds irreversibly and undergoes a 1, 2-addition reaction.
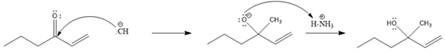
Explanation of Solution
A polar
In this reaction, the nucleophile adds irreversibly; hence this reaction undergoes direct addition (1, 2-addition). Here
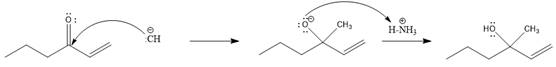
From the location of the electrophilic carbon, the major product for the given reaction is predicted, and the reaction mechanism is drawn.
(d)
Interpretation:
For the given reaction, the major product and the reaction mechanism is to be drawn.
Concept introduction:
The weak resonance contributor with separated charges generates a small partial positive charge on the beta carbon in the resonance hybrid. Thus, a nucleophile can attack at either the carbonyl carbon or the beta carbon. A polar
Answer to Problem 17.60P
The nucleophile adds irreversibly and undergoes a 1, 4-addition reaction.
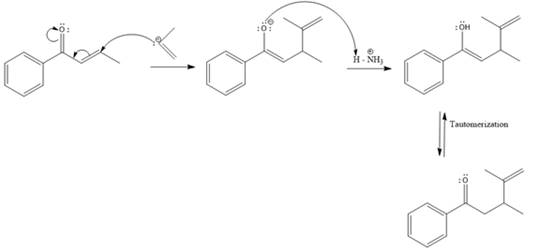
Explanation of Solution
The weak resonance contributor with separated charges generates a small partial positive charge on the beta carbon in the resonance hybrid. Thus, a nucleophile can attack at either the carbonyl carbon or the beta carbon. A polar
In this reaction, both carbonyl carbon as well as beta carbon are electrophilic. In this reaction, the nucleophile adds reversibly; therefore this reaction undergoes 1, 4-addition. In this reaction,
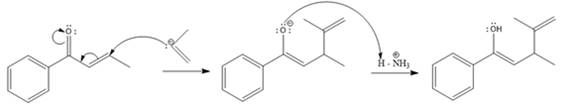
Here the enol tautomerizes back to the ketone via back to back proton transfer steps. It is shown below:
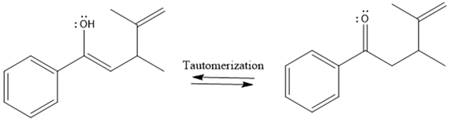
From the location of the electrophilic carbon, the major product for the given reaction is predicted, and the reaction mechanism is drawn.
(e)
Interpretation:
For the given reaction, the major product and the reaction mechanism is to be drawn.
Concept introduction:
The weak resonance contributor with separated charges generates a small partial positive charge on the beta carbon in the resonance hybrid. Thus, a nucleophile can attack at either the carbonyl carbon or the beta carbon. A polar
Answer to Problem 17.60P
The nucleophile adds irreversibly and undergoes a 1, 4-addition reaction.
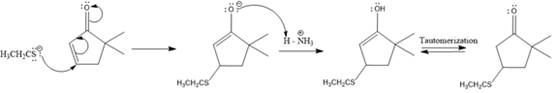
Explanation of Solution
The weak resonance contributor with separated charges generates a small partial positive charge on the beta carbon in the resonance hybrid. Thus, a nucleophile can attack at either the carbonyl carbon or the beta carbon. A polar
In this reaction, both carbonyl carbon as well as beta carbon are electrophilic. In this reaction, the nucleophile adds reversibly; therefore this reaction undergoes 1, 4-addition. In this reaction,
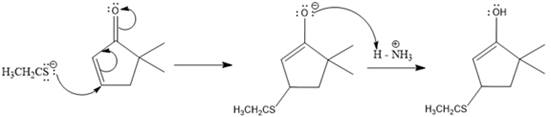
Here the enol tautomerizes back to the ketone via back to back proton transfer steps. It is shown below:
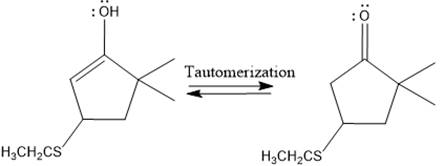
From the location of the electrophilic carbon, the major product for the given reaction is predicted, and the reaction mechanism is drawn.
Want to see more full solutions like this?
Chapter 17 Solutions
ORGANIC CHEMISTRY E-BOOK W/SMARTWORK5
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





