
Concept explainers
(a)
Interpretation:
The detailed mechanism for the given reaction is to be drawn, and the major product is to be predicted.
Concept introduction:
Lithium aluminium hydride (LAH) or
The chemical behaviour of deuterium (
Answer to Problem 17.43P
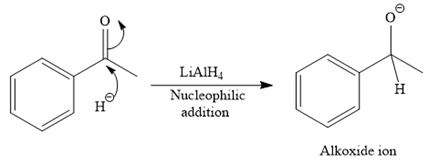
The detailed mechanism for the given reaction is
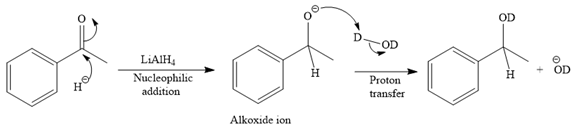
The major product of the given reaction:
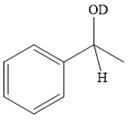
Explanation of Solution
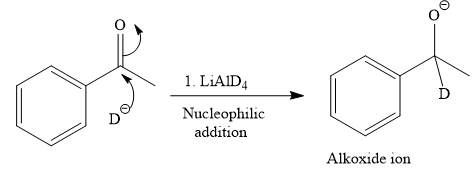
The given reaction is
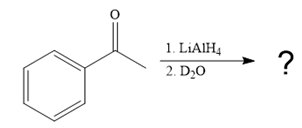
This alkoxide ion then attacks the deuterium (
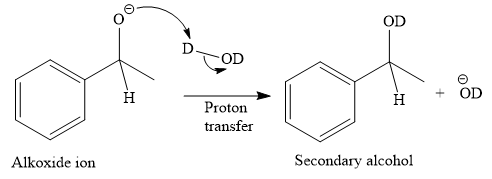
Thus, the final product of the given reaction is the secondary alcohol shown below:
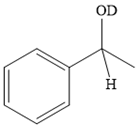
A ketone, when treated with a reducing agent such as Lithium aluminium hydride (LAH) or
(b)
Interpretation:
The detailed mechanism for the given reaction is to be drawn, and the major product is to be predicted.
Concept introduction:
Lithium aluminium hydride (LAH) or
The chemical behaviour of deuterium (
Answer to Problem 17.43P
The detailed mechanism for the given reaction is
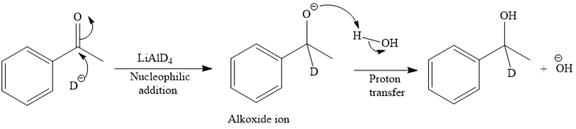
The major product of the given reaction:
Explanation of Solution
The given reaction is
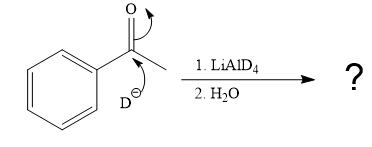
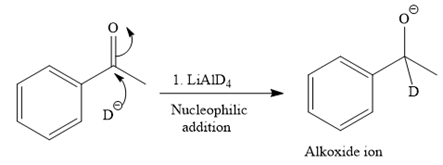
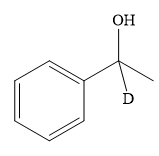
This alkoxide ion then attacks the proton (H) of water, which is the solvent used in the next step.
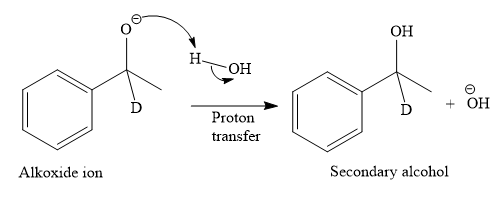
Thus, the final product of the given reaction is the secondary alcohol shown below:
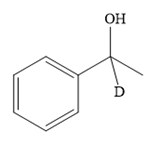
A ketone, when treated with a reducing agent such as Lithium aluminium hydride (LAH) or
(c)
Interpretation:
The detailed mechanism for the given reaction is to be drawn, and the major product is to be predicted.
Concept introduction:
Lithium aluminium hydride (LAH) or
The chemical behaviour of deuterium (
Answer to Problem 17.43P
The detailed mechanism for the given reaction is
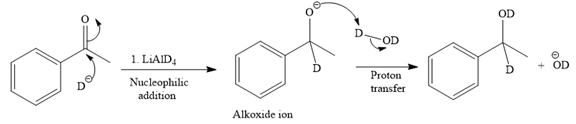
The major product of the given reaction:
Explanation of Solution
The given reaction is
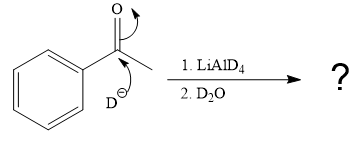
This alkoxide ion then attacks the deuterium (
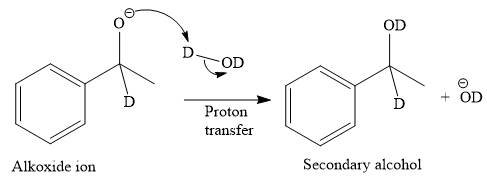
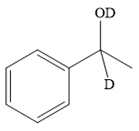
Thus, the final product of the given reaction is the secondary alcohol shown below:
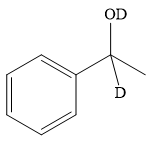
A ketone, when treated with a reducing agent such as Lithium aluminium hydride (LAH) or
Want to see more full solutions like this?
Chapter 17 Solutions
ORGANIC CHEMISTRY E-BOOK W/SMARTWORK5
- Complete the following reaction by mechanism E1 and E2. Present all steps and all possible products in each mechanism. See the image.arrow_forwardDraw a detailed mechanism of the following reactions and determine the major product:arrow_forwardPresent a detailed mechanism for the following reactions:arrow_forward
- Provide a detailed mechanism for the complete reactionarrow_forwardComplete all the reactions/show the products, and for each reaction clearly and thoroughly explain which mechanism (E1, E2, SN1, SN2) is predominant and how it effects the product formation.arrow_forwardPlease help with this problem. Please explain the full mechanism of the reaction. Thanks!arrow_forward
- A student attempted a substitution reaction by heating a solution of the iodide shown below in methanol and obtained mostly product A and a small amount of product B. Provide a detailed mechanism of forming both products using curved arrows.arrow_forwardSuggest a detailed mechanism for each of the reactions below. Explain why the originating products were formed.arrow_forwardComplete all the elimination reactions/show the products, and for each reaction clearly and thoroughly explain which mechanism (E1, E2, SN1, etc.) is predominant and how it effects the product formation.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





