
Concept explainers
Interpretation:
The name of the sugar that is formed after the digestion of starch is to be stated.
Concept introduction:
The compounds that are composed of carbon, hydrogen, and oxygen with empirical formula 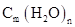 are known as carbohydrates. They are divided into three groups which are monosaccharides, oligosaccharides, and polysaccharides.
are known as carbohydrates. They are divided into three groups which are monosaccharides, oligosaccharides, and polysaccharides.
Monosaccharides are the simplest carbohydrates which cannot be hydrolyzed further. Examples of monosaccharides are glucose and fructose.
Oligosaccharides are the carbohydrates which can be hydrolyzed into two to ten monosaccharides. An example of oligosaccharide is glycolipids.
Polysaccharides are the carbohydrates which can hydrolyze into more than ten monosaccharides. For example, Starch.
Want to see the full answer?
Check out a sample textbook solution
Chapter 17 Solutions
Chemistry For Changing Times (14th Edition)
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





