
Concept explainers
Epoxide Rearrangements and the NIH Shift
This passage is about two seemingly unrelated aspects of
epoxide rearrangements
arene oxides
These two topics merge in an important biological transformation in which neither the
reactant nor the product is an epoxide

Epoxide rearrangements
In some epoxide ring-opening reactions
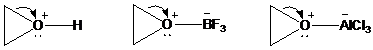
As positive charge develops on the ring carbon, one of the groups on the adjacent carbon migrates to it. This migration is assisted by electron
all of this occurs in the same transition state. Subsequent deprotonation gives an
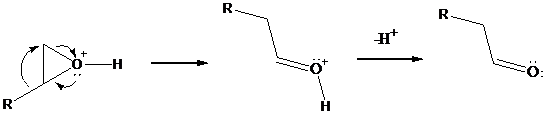
Overall, the reaction resembles the pinacol rearrangement of vicinal
Descriptive Passage and Interpretive Problems) and takes place under similar conditions.
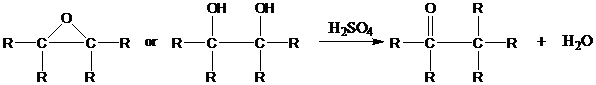
Arene Oxides
Aromatic rings are normally inert to the customary reagents that convert
arene oxides have been synthesized in the laboratory, often by indirect methods. Their chemical
reactivity resembles that of other epoxides.

The most striking thing about arene oxides is their involvement in biological processes. Enzymes
In the liver oxidize
The NIH shift
Although hydroxylation of phenylalanine to tyrosine looks like a typical electrophilic aromatic sub stitution, scientists at the U.S. National Institutes of Health discovered that the biochemical pathway combines epoxidation of the benzene ring followed by epoxide ring opening with rearrangement. This rearrangement, which is the biochemical analog of the pinacol

Acetanilide, which has pain-relieving properties, undergoes a biochemical oxidation
similar to that of the NIH shift that occurs with phenylalanine. The product formed from
acetanilide is itself a pain reliever. What is the structure of this substance (better known as
Tylenol)?
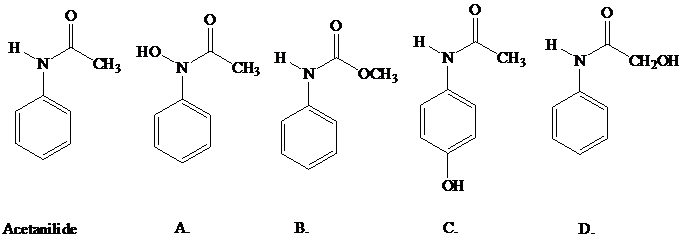
Want to see the full answer?
Check out a sample textbook solution
Chapter 17 Solutions
Organic Chemistry - Standalone book
- The following bicyclic ketone has two -carbons and three -hydrogens. When this molecule is treated with D2O in the presence of an acid catalyst, only two of the three -hydrogens exchange with deuterium. The -hydrogen at the bridgehead does not exchange. How do you account for the fact that two -hydrogens do exchange but the third does not? You will find it helpful to build models of the enols by which exchange of -hydrogens occurs.arrow_forwardwe know that ethers, such as diethyl ether and tetrahydrofuran, are quite resistant to the action of dilute acids and require hot concentrated HI or HBr for cleavage. However, acetals in which two ether groups are linked to the same carbon undergo hydrolysis readily, even in dilute aqueous acid. How do you account for this marked difference in chemical reactivity toward dilute aqueous acid between ethers and acetals?arrow_forwardConsider the tetracyclic aromatic compound drawn below, with rings labeled as A, B, C, and (a) Which of the four rings is most reactive in electrophilic aromatic substitution? (b) Which of the four rings is least reactive in electrophilic aromatic substitution? (c) What are the major produces) formed when this compound is treated with one equivalent of Br2?arrow_forward
- Benzene is one of the compounds used as octane enhancers in unleaded gasoline. It is manufactured by thecatalytic conversion of acetylene to benzene: 3C2 H2(g) ⇌ C6 H6(g). Which value of Kc would make this reactionmost useful commercially? Kc ≈ 0.01, Kc ≈ 1, or Kc ≈ 10. Explain your answerarrow_forwardThe carbocation electrophile in a Friedel-Crafts reaction can be generated in ways other than by reaction of an alkyl chloride with AlCl3. For example, reaction of benzene with 2-methylpropene in the presence of H3PO4 yields tert-butylbenzene. Draw a structure for the electrophile.arrow_forwardAll rearrangements we have discussed so far have involved generation of an electron-deficient carbon followed by a 1,2-shift of an atom or a group of atoms from an adjacent atom to the electron-deficient carbon. Rearrangements by a 1,2-shift can also occur following the generation of an electron-deficient oxygen. Propose a mechanism for the acid-catalyzed rearrangement of cumene hydroperoxide to phenol and acetone.arrow_forward
- A newer generation of antipsychotics, among them clozapine, are now used to treat the symptoms of schizophrenia. These drugs are more effective than earlier drugs in improving patient response in the areas of social withdrawal, apathy, memory, comprehension, and judgment. They also produce fewer side effects such as seizures and tardive dyskinesia (involuntary body movements). In the following synthesis of clozapine, Step 1 is an Ullmann coupling, a type of nucleophilic aromatic substitution that uses a copper catalyst. (a) Show how you might bring about formation of the amide in Step 2. (b) Propose a reagent for Step 3. (c) Propose a mechanism for Step 4. (d) Is clozapine chiral? If so, how many of the possible stereoisomers are formed in this synthesis?arrow_forwardIn the acid-catalyzed dehydration of 2-methyl-1-propanol, what carbocation would be formed if a hydride shift accompanied cleavage of the carbon–oxygen bond in the alkyloxonium ion? What ion would be formed as a result of a methyl shift? Which pathway do you think will predominate, a hydride shift or a methyl shift?arrow_forwardWhich of the following is NOT a correct statement about electrophilic aromatic substitution mechanism? Formation of a carbocation intermediate is the rate-determining step. The carbocation intermediate contains an sp3 hybridized carbon in the ring. The addition product is a frequent minor product. Aromaticity is regained by loss of H+ Benzene functions as nucleophile.arrow_forward
- Give reasons for the following: (i) Benzyl chloride is highly reactive towards the SN1 reaction. (ii) 2-bromobutane is optically active but 1-bromobutane is optically inactive. (iii) Electrophilic reactions in haloarenes occur slowly.arrow_forwardBenzene, C6H6 undergoes substitution reaction with concentrated nitricacid, HNO3 to produce compound L. The reaction of compound L withbromine, Br2 in the presence of iron tribromide, FeBr3 produced compoundM. Benzene also undergoes Fridel-crafts alkylation reaction withchloroethane, CH3CH2Cl using catalyst N to produce compound P (i) Draw the structural formula of L, M and P (ii) State catalyst N. (iii) Show the formation of electrophile that will be reacted with benzene for theformation of compound P.arrow_forwardWhen the compound shown below undergoes acid-catalyzed dehydration, a ring expansionoccurs to give the fused-ring product. What type of intermediate is formed in this reaction?Explain the ring expansion in terms of the reaction mechanismarrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
