
Organic Chemistry
8th Edition
ISBN: 9781305580350
Author: William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 22, Problem 22.62P
A newer generation of antipsychotics, among them clozapine, are now used to treat the symptoms of schizophrenia. These drugs are more effective than earlier drugs in improving patient response in the areas of social withdrawal, apathy, memory, comprehension, and judgment. They also produce fewer side effects such as seizures and tardive dyskinesia (involuntary body movements). In the following synthesis of clozapine, Step 1 is an Ullmann coupling, a type of nucleophilic
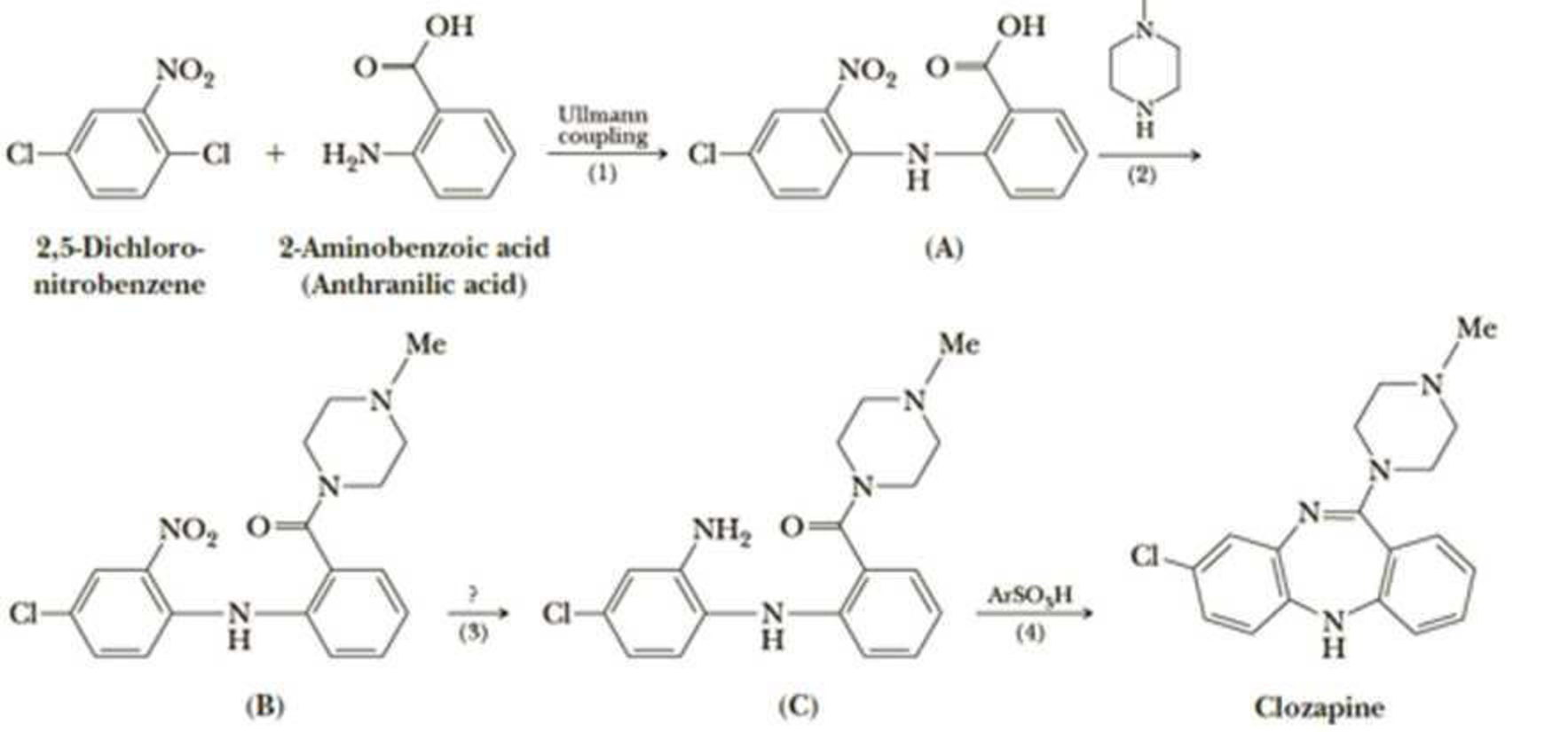
- (a) Show how you might bring about formation of the amide in Step 2.
- (b) Propose a reagent for Step 3.
- (c) Propose a mechanism for Step 4.
- (d) Is clozapine chiral? If so, how many of the possible stereoisomers are formed in this synthesis?
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
a. Compound X is benzene, Y is acetic anhydride acid. Complete the following scheme and determine Z!
b. Determine which reagents except acetic acid anhydrides can replace Y!
Benzene is one of the compounds used as octane enhancers in unleaded gasoline. It is manufactured by thecatalytic conversion of acetylene to benzene: 3C2 H2(g) ⇌ C6 H6(g). Which value of Kc would make this reactionmost useful commercially? Kc ≈ 0.01, Kc ≈ 1, or Kc ≈ 10. Explain your answer
A key step in the synthesis of naproxen, an NSAID more commonly known by its brand name, Aleve (Section 3.9), is a coupling reaction of 2-bromo6-methoxynaphthalene to form 2-methoxy-6-vinylnaphthalene. Show three different coupling reactions, and the required reagents, that could be used to carry out this step.
Chapter 22 Solutions
Organic Chemistry
Ch. 22.1 - Prob. 22.1PCh. 22.1 - Write a structural formula for the product from...Ch. 22.1 - Prob. 22.3PCh. 22.2 - Prob. 22.4PCh. 22.2 - Predict the major produce(s) of each electrophilic...Ch. 22.3 - In SN2 reactions of haloalkanes, the order of...Ch. 22 - Prob. 22.8PCh. 22 - Prob. 22.9PCh. 22 - Addition of m-xylene to the strongly acidic...Ch. 22 - Addition of tert-butylbenzene to the strongly...
Ch. 22 - What product do you predict from the reaction of...Ch. 22 - Other groups besides H+ can act as leaving groups...Ch. 22 - Prob. 22.14PCh. 22 - Prob. 22.15PCh. 22 - Prob. 22.16PCh. 22 - Prob. 22.17PCh. 22 - Suggest a reason why the nitroso group, N=O, is...Ch. 22 - Prob. 22.19PCh. 22 - Prob. 22.20PCh. 22 - The following molecules each contain two aromatic...Ch. 22 - Prob. 22.22PCh. 22 - Prob. 22.23PCh. 22 - The insecticide DDT is prepared by the following...Ch. 22 - Prob. 22.25PCh. 22 - Prob. 22.26PCh. 22 - Prob. 22.27PCh. 22 - Prob. 22.28PCh. 22 - Prob. 22.29PCh. 22 - Prob. 22.32PCh. 22 - Show how to prepare each compound from...Ch. 22 - Prob. 22.34PCh. 22 - Show reagents and conditions to bring about the...Ch. 22 - Prob. 22.36PCh. 22 - Propose a synthesis for each compound from...Ch. 22 - The first widely used herbicide for the control of...Ch. 22 - The first widely used herbicide for the control of...Ch. 22 - Prob. 22.40PCh. 22 - Prob. 22.41PCh. 22 - Prob. 22.42PCh. 22 - Prob. 22.43PCh. 22 - Cancer of the prostate is the second leading cause...Ch. 22 - Prob. 22.45PCh. 22 - Prob. 22.46PCh. 22 - Prob. 22.47PCh. 22 - When certain aromatic compounds are treated with...Ch. 22 - Prob. 22.49PCh. 22 - Following is the structure of miconazole, the...Ch. 22 - Prob. 22.51PCh. 22 - Prob. 22.52PCh. 22 - Prob. 22.53PCh. 22 - Show how the antidepressant venlafaxine (Effexor)...Ch. 22 - Prob. 22.57PCh. 22 - Given this retrosynthetic analysis, propose a...Ch. 22 - Prob. 22.59PCh. 22 - Prob. 22.60PCh. 22 - Prob. 22.61PCh. 22 - A newer generation of antipsychotics, among them...Ch. 22 - Prob. 22.63P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- gas, Cl¬CH2CH2¬S¬CH2CH2¬Cl, was used as a poisonous chemical agentin World War I. Mustard gas is much more toxic than a typical primary alkyl chloride. Itstoxicity stems from its ability to alkylate amino groups on important metabolic enzymes,rendering the enzymes inactive.(a) Propose a mechanism to explain why mustard gas is an exceptionally potent alkylatingagent.(b) Bleach (sodium hypochlorite, NaOCl, a strong oxidizing agent) neutralizes and inactivates mustard gas. Bleach is also effective on organic stains because it oxidizes coloredcompounds to colorless compounds. Propose products that might be formed by thereaction of mustard gas with bleach.arrow_forwardConsider the tetracyclic aromatic compound drawn below, with rings labeled as A, B, C, and (a) Which of the four rings is most reactive in electrophilic aromatic substitution? (b) Which of the four rings is least reactive in electrophilic aromatic substitution? (c) What are the major produces) formed when this compound is treated with one equivalent of Br2?arrow_forwardShow an actual arrow-pushing mechanism for the transformation below, and briefly explain the observed regioselectivity.arrow_forward
- Show how the following compounds will be synthesized, beginning with benzene or toluene and any reagents required. Assume para, in ortho, para mixtures, are the main ingredients (and separable from ortho). 1) p-bromobenzene sulfonic acid 2) 1-phenypentane 3) m-chloronitrobenzene 4) o-chlorobenzoic acidarrow_forwardChoose the right reagent or series of reagents from the ones listed below to prepare 2-pentanone from acetylene. NaNH2NaNH2 followed by CH3CH2CH2BrCH3CH2CH2Br, then disiamylborane followed by H2O2H2O2, HO−HO− H2H2, Lindlar followed by H2OH2O, H+H+ NaNH2NaNH2 and CH3CH2CHOCH3CH2CHO NaNH2NaNH2 followed by H2OH2O, H+H+ NaNH2NaNH2 followed by CH3CH2CH2BrCH3CH2CH2Br, then H2OH2O, H2SO4H2SO4, HgSO4 Choose one.arrow_forwardodine monochloride, ICI, is a reagent whose reactivity mirrors that of other dihalogens (X₂) with nucleophilic πt bonds. Using curved arrows to show the flow of electrons, draw a plausible mechanism for the following transformation. Make sure that your mechanism accounts for the correct stereoselectivity.arrow_forward
- A key step in the synthesis of naproxen, an NSAID more commonly known by its brand name, Aleve (Section 3.9), is a coupling reaction of 2-bromo-6-methoxynaphthalene to form 2-methoxy-6-vinylnaphthalene. Show three different coupling reactions, and the required reagents, that could be used tocarry out this step.arrow_forwardPropose a mechansim for the following transformationarrow_forwardWhat is the missing reagent needed to perform the following transformation? Assume mildly acidic catalysis. a) CH3NH2 b) CH2=NH c) (CH3)2NH d) CH3CH2NH2arrow_forward
- In the acid-catalyzed dehydration of 2-methyl-1-propanol, what carbocation would be formed if a hydride shift accompanied cleavage of the carbon–oxygen bond in the alkyloxonium ion? What ion would be formed as a result of a methyl shift? Which pathway do you think will predominate, a hydride shift or a methyl shift?arrow_forwardPropose an efficient synthesis for the following transformation: 1-butyne to trans-3,4-dibromohexaneThe transformation above can be performed with some reagent or combination of the reagents listed below. Give the necessary reagent(s) in the correct order, as a string of letters (without spaces or punctuation, such as “EBF”). If there is more than one correct solution, provide just one answer. A B C Br2 NaNH2 Na, NH3 (l) D E F dilute H2SO4 H2, Lindlar's catalyst MeI G H I 1) BH3·THF2) H2O2, H2O 1) xs NaNH22) H2Oarrow_forwardProvide suitable reagents to effect the following transformations.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
Alcohols, Ethers, and Epoxides: Crash Course Organic Chemistry #24; Author: Crash Course;https://www.youtube.com/watch?v=j04zMFwDeDU;License: Standard YouTube License, CC-BY