
Concept explainers
(a)
Interpretation:
The name of the given compound has to be determined.
Concept introduction:
In chemistry structure is the arrangement of
According to
The order of priority is,
Carboxylic acid: One
Amide: One
Depending on the number of carbon side chain of the amide, different types of amides can form.
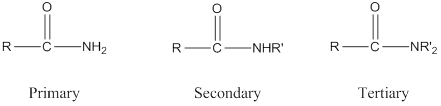
Primary amides can be named in the IUPAC system in several ways,
For simple amides the suffix – amide is added to the name of the alkyl substituent.
The suffix- amide can be used in place of the final –e in the name of the parent compound.
For a secondary amides an N prefixes the compound giving the shorter carbon chain and its chain prefix name.
For a tertiary amides an N, N prefixes the compound giving the two shorter carbon chains and their side chain prefix names.
(b)
Interpretation:
The name of the given compound has to be determined.
Concept introduction:
In chemistry structure is the arrangement of chemical bonds between atoms in a molecule, specifically which atoms are chemically bonded to what other atoms with what kind of chemical bond.
According to IUPAC nomenclature, the naming of compound is determined by the priority of the functional group if more than one functional group is present. The carbon attached to the functional group having most priority should get the least number while naming the compound.
The order of priority is,
Carboxylic acid: One
Amide: One
Depending on the number of carbon side chain of the amide, different types of amides can form.
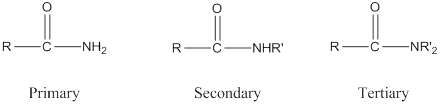
Primary amides can be named in the IUPAC system in several ways,
For simple amides the suffix – amide is added to the name of the alkyl substituent.
The suffix- amide can be used in place of the final –e in the name of the parent compound.
For a secondary amides an N prefixes the compound giving the shorter carbon chain and its chain prefix name.
For a tertiary amides an N, N prefixes the compound giving the two shorter carbon chains and their side chain prefix names.
(c)
Interpretation:
The name of the given compounds has to be determined.
Concept introduction:
In chemistry structure is the arrangement of chemical bonds between atoms in a molecule, specifically which atoms are chemically bonded to what other atoms with what kind of chemical bond.
According to IUPAC nomenclature, the naming of compound is determined by the priority of the functional group if more than one functional group is present. The carbon attached to the functional group having most priority should get the least number while naming the compound.
The order of priority is,
Carboxylic acid: One
Amide: One
Depending on the number of carbon side chain of the amide, different types of amides can form.
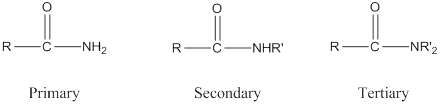
Primary amides can be named in the IUPAC system in several ways,
For simple amides the suffix – amide is added to the name of the alkyl substituent.
The suffix- amide can be used in place of the final –e in the name of the parent compound.
For a secondary amides an N prefixes the compound giving the shorter carbon chain and its chain prefix name.
For a tertiary amides an N, N prefixes the compound giving the two shorter carbon chains and their side chain prefix names.
Want to see the full answer?
Check out a sample textbook solution
Chapter 17 Solutions
Modified Mastering Chemistry With Pearson Etext -- Standalone Access Card -- For Fundamentals Of General, Organic, And Biological Chemistry (8th Edition)
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON





