
To draw: the dot diagram for any one of the molecules out of
Explanation of Solution
Introduction:
Electron dot structures are diagrams demonstrating the bonding of the molecular atoms to the lone pairs of electrons that can occur in the molecule. It is also known as Lewis diagram.
Since nitrogen molecule is chemically inert and also stable, because single nitrogen is unstable due to lack electrons in valence shell. That’s why, if two nitrogen shares their valence electrons, then it will become stable and their octet will also get completed.
Electron dot diagram for
The atomic number of Nitrogen is 7 and its electronic configuration is
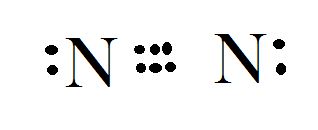
Electron dot diagram for
The atomic number of Nitrogen is 6 and its electronic configuration is
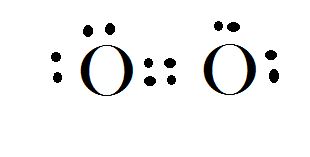
Electron dot diagram for
The atomic number of Nitrogen is 1 and its electronic configuration is
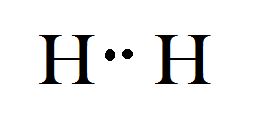
Conclusion:
Hence, diagrams for
Chapter 18 Solutions
Glencoe Physical Science 2012 Student Edition (Glencoe Science) (McGraw-Hill Education)
Additional Science Textbook Solutions
Sears And Zemansky's University Physics With Modern Physics
Introduction to Electrodynamics
Physics for Scientists and Engineers with Modern Physics
The Cosmic Perspective (8th Edition)
Physics: Principles with Applications
Life in the Universe (4th Edition)
 College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON
University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press
Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley
Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON
College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON





