
Concept explainers
Interpretation:
The stereochemistry of the pyruvate reduction shown is to be stated. Whether NADH lose its pro-R or pro-S hydrogen is to be stated. Whether the addition of hydrogen occurs to the Si face or Re face of pyruvate is also to be stated.
Concept introduction:
When an achiral molecule such as
In addition a compound with a sp3 hybridized carbon also is said to be prochiral if by changing one of the attached groups it becomes a chiral centre. If the prochiral centre has two identical atoms, then that atom whose replacement leads to a R chirality centre is said to be pro R and that atom whose replacement leads to a S chirality centre is said to be pro S.
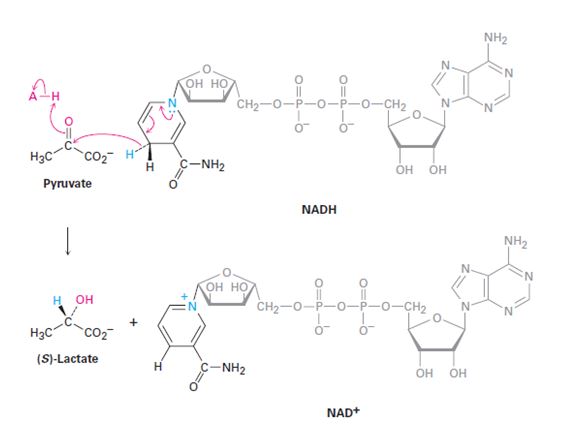
To show:
The stereochemistry of the pyruvate reduction to (s)-lactate shown and to state whether NADH lose its pro-R or pro-S hydrogen and whether the addition of hydrogen occurs to the Si face or Re face of pyruvate.
Trending nowThis is a popular solution!

Chapter 19 Solutions
ORGANIC CHEMISTRY-EBOOK>I<
- Draw the structure(s) for the major final product(s) formed in the following reaction sequence. CN s༠༣ Cl, FeCl. 3 H,SO Click and drag to start drawing a structure. lo 用 8 iYB & {EE□arrow_forwardWhich is the reductant and oxidant in the reaction? S2O32- + I2 → I- + S4O62 I2 is the reductant; S2O32- is the oxidant I- is the reductant; S4O62- is the oxidant S2O32- is the reductant; I2 is the oxidant S4O62- is the reductant; I- is the oxidantarrow_forward13-29 Show that if you add Steps 2a and 2b of the radical- chain mechanism for the autoxidation of a fatty acid hydrocarbon chain, you arrive at the following net equation: H I —CH2CH=CH—CH— + 0—0 • » Section of a fatty acid Oxygen hydrocarbon chain O—O—H I —CH2CH=CH—CH— A hydroperoxidearrow_forward
- Draw the orgamic molecule(s) which is(are) formed in the following reaction. Do not include molecules like H20 or HCl. (You have 2 chances until the answer will be given; you have already tried 0 times) он H, CH K2Cr,O,/ H* H,C CH C H, H,arrow_forward6 How do you visualize stereochemistry? What kind of methods and tricks do you use to visualize these in the 3-D space? Trends and things you have noticed during practice that you can now use as shortcuts.arrow_forwardSodium borohydride (NABH4) is said to be a chemoselective reducing agent. Which of the following best describes what this term means? O 1 A reaction that operates exclusively on one functional group in the presence of other functional groups. A reaction that operates on one functional group at a time and is shut down 2) when other functional groups are present. O 3) A reaction that does not operate at all in the presence of a functional group. OA reaction that operates on multiple functional groups in the presence of 4) multiple functional groups.arrow_forward

 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning

