
a)
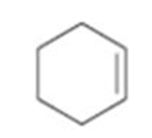
Interpretation:
How to prepare cyclohexene from 2-cyclohexenone is to be stated.
Concept introduction:
Wolf-Kishner reduction rection can be used to prepare cyclohexene from 2-cyclohexenone.
To state:
How to prepare cyclohexene from 2-cyclohexenone.
b)
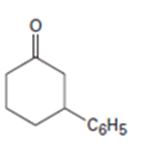
Interpretation:
How to prepare 3-phenylcyclohexanone from 2-cyclohexenone is to be stated.
Concept introduction:
By treating 2-cyclohexenone with lithiumdiphenylcopper and acidifying the product formed 3-phenylcyclohexanone can be prepared, as the organolithiumcopper reagents are good reagents for the conjugate addition to α,β- unsaturated
To state:
How to prepare 3-phenylcyclohexanone from 2-cyclohexenone.
c)
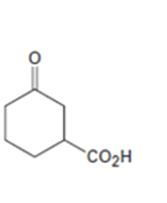
Interpretation:
How to prepare the ketoacid shown from 2-cyclohexenone is to be stated.
Concept introduction:
An easily oxidizable group is introduced to C3 of 2-cyclohexenone by a conjugate addition using lithiumdialkylcopper reagent. The resulting product can then be oxidized to a
To state:
How to prepare the keto acid shown from 2-cyclohexenone.
d)
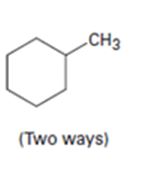
Interpretation:
How to prepare methylcyclohexane from 2-cyclohexenone (two ways) is to be shown.
Concept introduction:
An alkyl group can be introduced into 2-cyclohexenone by treating it with lithiumdialkyl reagent(conjugate addition takes place). The keto group can be reduced to CH2 by Wolff-Kishner reduction.
Another way is to replace the C =O in 2-cyclohexenone by C =CH2 by a Wittig reaction first and then reducing it to C-CH3 by H2, Pd/C.
To state:
How to prepare methylcyclohexane from 2-cyclohexenone.
Trending nowThis is a popular solution!

Chapter 19 Solutions
ORGANIC CHEMISTRY-EBOOK>I<
- Give the major product of the reaction shown below. ? Cl₂ H₂O I CI IV Compound I O Compound IV Compound II Compound V Compound III OH LOH do you g V OH LOHarrow_forwardWhat is the expected major product of this reaction? OH H₂N I H₂N H₂N 85% H3PO4, A H₂N ||| ? H₂N IVarrow_forwardWhat alkene is needed to synthesize each 1,2-diol using [1] OsO4 followed by NaHSO3 in H2O; or [2] CH3CO3H followed by −OH in H2O?arrow_forward
- C. How would you synthesize the given target compound in multistep syntheses? 2 НО CO₂H CH3arrow_forwardName the following organic compound: CH3 S CH CH3 CH 0 CH, CH, 3-methylsulfide-2-ethoxy butane 2-ethoxy-3-methylthio butane O 3-ethoxy-2-methylthio butane O 2-methylsulfide-3-ethoxy butane O 2-ethoxy-3-methylsulfide butanearrow_forwardHow could you prepare the following compounds from the given starting materials?arrow_forward
- 2) How would you synthesize the following compounds from cyclohexanone? CH₂Br CH₂C6H5 A Br B C D CH,CH2CO2Harrow_forwardWhat is the major organic product obtained from the following reaction? 1. ВНз CH;CH,CH;CH,-cEC-H 2. H,O, NaOH O hexanone O hexanal O 2-hexanol O cis-2-hexenearrow_forwardWhat are the reagents A and B in the scheme below? Br CH3 в CH,CH,CH,C-CH, A HgC CHy H;C CHarrow_forward
- 11 R - C - R' Ketone ·0: ון + CH₂ CH₂-0 - CH₂ CH 3 R - CH + CH₂ CH₂-- CH ₂ CH 3 Aldehyde n. R-OH + + CH3 CH2 - Ộ - CH2 CH3 ? n. Alcohol ? R-C-OH + CH₂ CH₂ - 0 - CH₂ CH₂ ? 2. Carboxylic acid H 1 R-N-H + CH₂CH₂- Ő - CH₂CH₂ Amine ?arrow_forwardWhich of the following reactions does not proceed? O Dehydration of 2-propanol with H;SO, catalyst Hydrogenation of benzene with H2/Pt o Oxidation of 2-propanol by H,Cro O Hydrobromination of 2-butene with HBr O Hydration of cyclohexene with H2O/H2SO4arrow_forward6-40 Give the substitution products expected from solvolysis of each compound by heating in ethanol. (a) (b) (c) Br (d) Br CI CH3 Brarrow_forward
