
Concept explainers
Interpretation:
Molecular geometry and electron geometry about nitrogen atom in the acetone imine molecule is to be predicted.
Concept introduction:
Electron geometry and molecular geometry of molecules is determined by using Valence shell electron pair repulsion (VSEPR) theory. According to VSEPR theory, electron geometry describes the orientation of the electron groups about a particular atom, and molecular geometry describes the arrangement of atoms about a particular atom.
Number of electron pairs describes the electron and molecular geometry. If all the electron pairs are bonds, then molecular geometry is the same as electron geometry. Electron geometry is different from molecular geometry if some electron pairs are present as lone pairs.
Electron geometry and molecular geometry from the number of electron pairs are as follows:
| Number of electron pairs |
Number of Bonds |
Number of Lone pair |
Electron geometry | Molecular Geometry |
| 2 | 2 | 0 | Linear | Linear |
| 3 | 3 | 0 | Trigonal planar | Trigonal planer |
| 3 | 2 | 1 | Trigonal planar | Bent |
Answer to Problem 2.1P
Electron geometry about N atom in acetone imine molecule geometry is trigonal planar and molecular geometry is bent.
Explanation of Solution
The given acetone imine molecule is
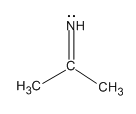
In acetone imine molecule, there are total three groups of electrons about N atom. There is one double bond, one single bond, and one lone pair around N atom, that is, all electron pairs are not involved in bond formation, and hence, molecular geometry and electron geometry are different.
There are total three electron groups, hence, the electron geometry about N atom is trigonal planar. The molecular geometry is bent.
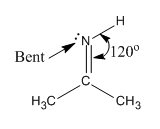
Electron geometry and molecular geometry about N atom is predicted from the number of electron pairs present around the atom in the given molecule.
Want to see more full solutions like this?
Chapter 2 Solutions
ORGANIC CHEMISTRY E-BOOK W/SMARTWORK5
- Based on your answer to below Problem, do you thinkthe compound shown here should have a significantdipole moment? If so, in which direction does it point? The molecule shown here has quite a large dipole, asindicated in its electrostatic potential map. Explain why.Hint: Consider various resonance structures.arrow_forwardplease solve the problem following the given steps in the second picturearrow_forwardall one problem, fill in the blanks, draw the arrowsarrow_forward
- In this molecule’s other chair conformation, how many (non H) axial positions are there?arrow_forwardProblem What amount (mol) of each ion is in each solution?(a) 5.0 mol of ammonium sulfate dissolved in water(b) 78.5 g of cesium bromide dissolved in water(c) 7.42×1022 formula units of copper(II) nitrate dissolved in water(d) 35 mL of 0.84 M zinc chloridePlan We write an equation that shows 1 mol of compound dissociating into ions. (a) We multiply the number of moles of ions by 5.0. (b) We first convert grams to moles. (c) We first convert formula units to moles. (d) We first convert molarity and volume to moles.arrow_forwardWhat is the hybridizarion of the atom labeled with arrow in each of the following molecules?arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
