
(a)
Interpretation:
The product of the reaction between
Concept introduction:
Carboxylic acid derivatives undergo acyl group substitution reactions when treated with appropriate nucleophiles. The reaction occurs via nucleophilic addition-elimination involving a tetrahedral intermediate. It may also involve proton transfer step(s). The reaction occurs if the possible product is more stable than the reactant. If the two are of comparable stability, the reaction will occur reversibly. The order of increasing stability of acid derivatives is
Answer to Problem 20.35P
The product of the given reaction is
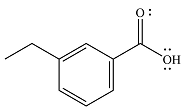
The complete mechanism of the reaction is
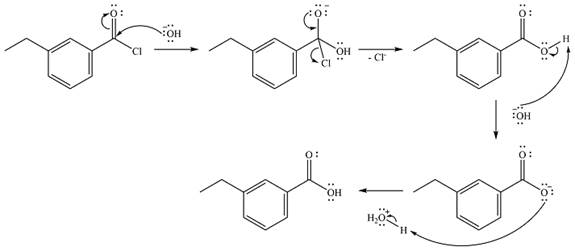
Explanation of Solution
The given reactant is NaOH, followed by
Thus, the product of the reaction will be

The reaction will start with the nucleophilic addition of the hydroxide ion from NaOH, producing a tetrahedral intermediate.
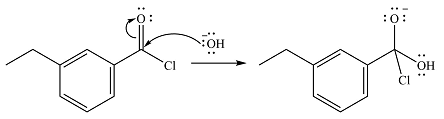
In the second step, one lone pair from the negatively charged oxygen will move back to the carbon to reform the carbonyl group and force the chlorine atom to leave as a chloride ion. This step will produce the corresponding carboxylic acid, but under the strongly basic conditions, it will be irreversibly deprotonated to the carboxylate anion.
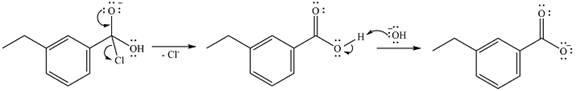
The addition of the acid (
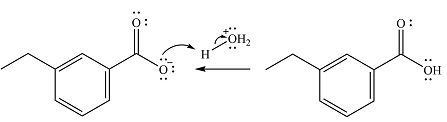
Thus, the complete mechanism can be drawn as
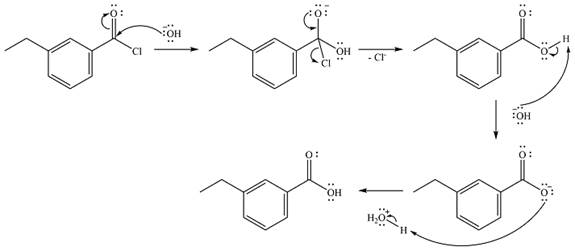
The product of the reaction and its mechanism are determined based on the relative stability of the product and the nucleophilic addition-elimination mechanism.
(b)
Interpretation:
The product of the reaction between
Concept introduction:
Carboxylic acid derivatives undergo acyl group substitution reactions when treated with appropriate nucleophiles. The reaction occurs via nucleophilic addition-elimination involving a tetrahedral intermediate. It may also involve proton transfer step(s). The reaction occurs if the possible product is more stable than the reactant. If the two are of comparable stability, the reaction will occur reversibly. The order of increasing stability of acid derivatives is
Answer to Problem 20.35P
The product of the given reaction is
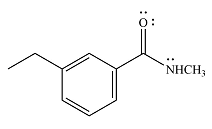
The complete mechanism of the reaction is
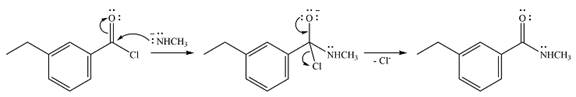
Explanation of Solution
The given reactant is
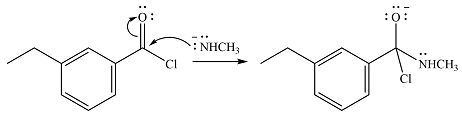
One lone pair on the negatively charged oxygen will move back to the carbon to reform the carbnyl group and eliminate chloride to form the final product.
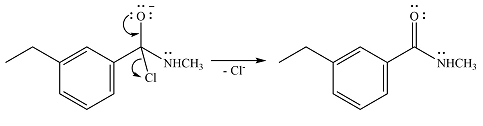
Thus, the product of the reaction will be

And the complete mechanism can be drawn as

The product of the reaction and its mechanism are determined based on the relative stability of the product and the nucleophilic addition-elimination mechanism.
(c)
Interpretation:
The product of the reaction between
Concept introduction:
Carboxylic acid derivatives undergo acyl group substitution reactions when treated with appropriate nucleophiles. The reaction occurs via nucleophilic addition-elimination involving a tetrahedral intermediate. It may also involve proton transfer step(s). The reaction occurs if the possible product is more stable than the reactant. If the two are of comparable stability, the reaction will occur reversibly. The order of increasing stability of acid derivatives is
Answer to Problem 20.35P
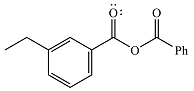
The product of the given reaction is
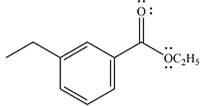
The complete mechanism of the reaction is
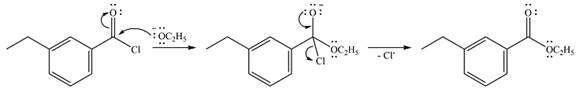
Explanation of Solution
The given reactant is
Therefore, the product of the reaction will be

In the first step, the incoming nucleophile will add to the carbonyl carbon, producing a tetrahedral intermediate.
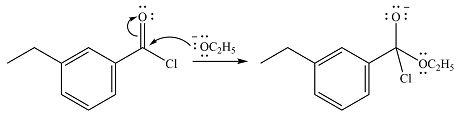
The lone pair on the negativey charged oxygen will move back toward the carbon to reform the carbonyl group and eliminate chloride to form the final product, an ester.
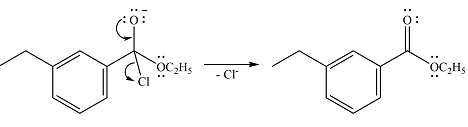
Thus, the complete mechanism can be drawn as
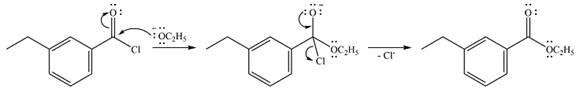
The product of the reaction and its mechanism are determined based on the relative stability of the product and the nucleophilic addition-elimination mechanism.
(d)
Interpretation:
The product of the reaction between
Concept introduction:
Carboxylic acid derivatives undergo acyl group substitution reactions when treated with appropriate nucleophiles. The reaction occurs via nucleophilic addition-elimination involving a tetrahedral intermediate. It may also involve proton transfer step(s). The reaction occurs if the possible product is more stable than the reactant. If the two are of comparable stability, the reaction will occur reversibly. The order of increasing stability of acid derivatives is
Answer to Problem 20.35P
The product of the given reaction is
The complete mechanism of the reaction is
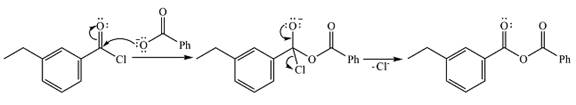
Explanation of Solution
The given reactant is
Therefore, the product of the reaction will be

In the first step, the nucleophile will add to the carbonyl carbon to produce a tetrahedral intermediate.
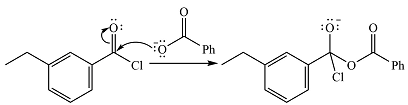
In the second step, one lone pair of the negatively charged oxygen will move back to the carbon to reform the carbonyl group and eliminate chloride to form the final product.
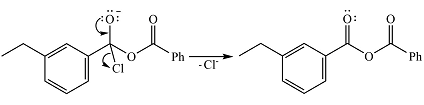
Thus, the complete mechanism can be drawn as

The product of the reaction and its mechanism are determined based on the relative stability of the product and the nucleophilic addition-elimination mechanism.
(e)
Interpretation:
The product of the reaction between
Concept introduction:
Carboxylic acid derivatives undergo acyl group substitution reactions when treated with appropriate nucleophiles. The reaction occurs via nucleophilic addition-elimination involving a tetrahedral intermediate. It may also involve proton transfer step(s). The reaction occurs if the possible product is more stable than the reactant. If the two are of comparable stability, the reaction will occur reversibly. The order of increasing stability of acid derivatives is
Answer to Problem 20.35P
There will be no reaction.
Explanation of Solution
The given reactant is
Therefore, the reaction will not occur.
Nucleophilic addition-elimination cannot occur since the given reactant is not a source of a nucleophile.
(f)
Interpretation:
The product of the reaction between
Concept introduction:
Carboxylic acid derivatives undergo acyl group substitution reactions when treated with appropriate nucleophiles. The reaction occurs via nucleophilic addition-elimination involving a tetrahedral intermediate. It may also involve proton transfer step(s). The reaction occurs if the possible product is more stable than the reactant. If the two are of comparable stability, the reaction will occur reversibly. The order of increasing stability of acid derivatives is
Answer to Problem 20.35P
The reaction will not occur.
Explanation of Solution
The given reactant is
Therefore, there will be no reaction.
Nucleophilic addition-elimination is not possible in this case as the nucleophile is weak and does not add to a carbonyl carbon.
Want to see more full solutions like this?
Chapter 20 Solutions
ORG.CHEM W/TEXT+SOLU.MANUAL
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





