
Concept explainers
(a)
Interpretation:
The product with detailed mechanism for the reaction between methyl benzoate and
Concept introduction:
An ester can be converted to a primary alcohol by reacting with
Answer to Problem 20.46P
The product with detailed mechanism for the reaction between methyl benzoate and
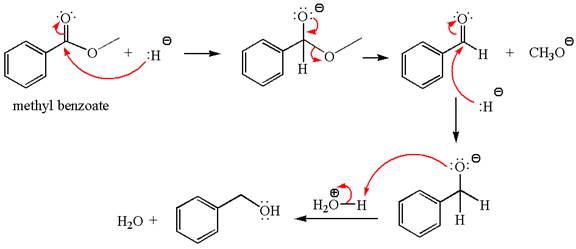
Explanation of Solution
The equation for the reaction of methyl benzoate with
Methyl benzoate is an ester, which, on reaction with
The product with detailed mechanism for the given reaction is drawn based on the reactivity of
(b)
Interpretation:
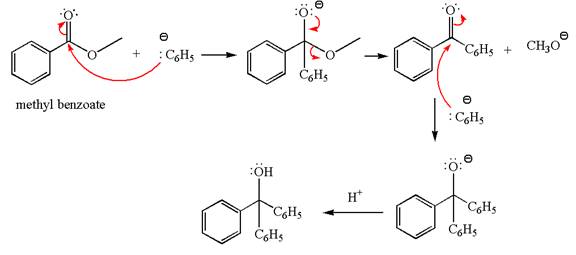
The product with detailed mechanism for the reaction between methyl benzoate and
Concept introduction:
An ester can be converted to a tertiary alcohol by reacting it with
Answer to Problem 20.46P
The product with detailed mechanism for the reaction between methyl benzoate and
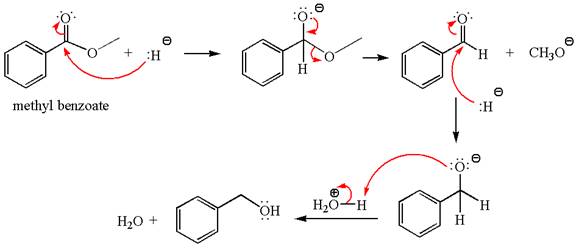
Explanation of Solution
The equation for the reaction of methyl benzoate with
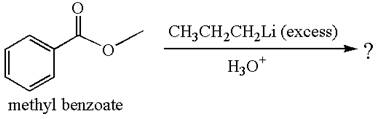
Methyl benzoate is an ester, which, on reaction with
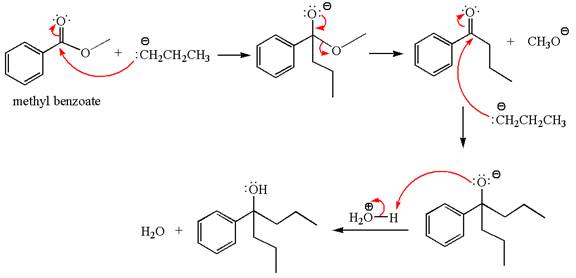
The product with detailed mechanism for the given reaction is drawn based on the reactivity of
(c)
Interpretation:
Whether methyl benzoate can react with
Concept introduction:
The reagent
Answer to Problem 20.46P
Mmethyl benzoate cannot react with
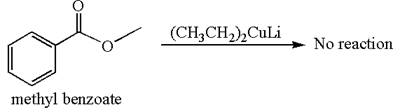
Explanation of Solution
The equation for the reaction of methyl benzoate with
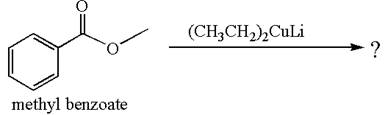
Methyl benzoate is an ester and
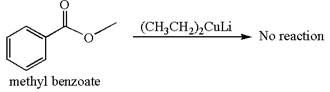
It is determined that no reaction occurs based on the reactivity of
(d)
Interpretation:
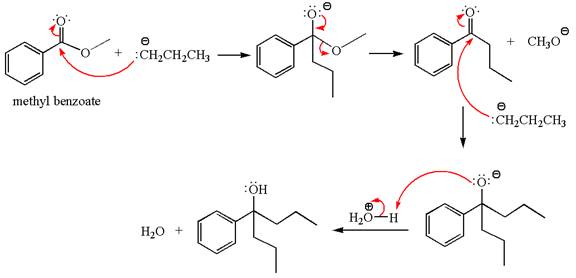
The product with detailed mechanism for the reaction between methyl benzoate and
Concept introduction:
An ester can be converted to a tertiary alcohol by reacting it with
Answer to Problem 20.46P
The product with detailed mechanism for the reaction between methyl benzoate and
Explanation of Solution
The equation for the reaction of methyl benzoate with
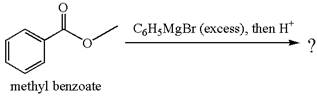
Methyl benzoate is an ester, which, on reaction with

The product with detailed mechanism for the given reaction is drawn based on the reactivity of
(e)
Interpretation:
The product with detailed mechanism for the reaction between methyl benzoate and
Concept introduction:
An ester can be reduced to aldehyde without reducing it further to alcohol using a specific reagent such as
Answer to Problem 20.46P
The product with detailed mechanism for the reaction between methyl benzoate and
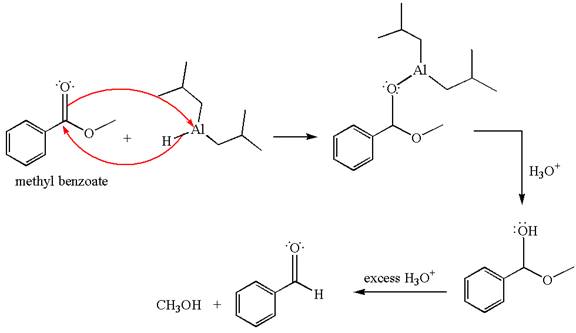
Explanation of Solution
The equation for the reaction of methyl benzoate with
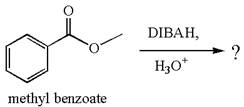
Methyl benzoate is an ester, which, on reaction with
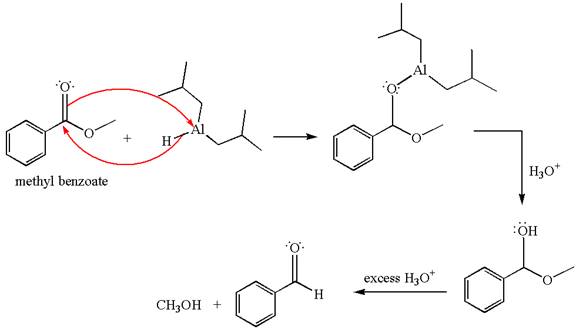
The product with detailed mechanism for the given reaction is drawn based on the reactivity of
Want to see more full solutions like this?
Chapter 20 Solutions
ORG.CHEM W/TEXT+SOLU.MANUAL
- Provide the mechanism and products of the reactionsarrow_forwardprovide a mechanism for these reactionsarrow_forwardProvide the whole reaction mechanism (generation of electrophile, nucleophile, bond formation, bond breaking and movement of arrows) and the final product of the following reactions:arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





