
Interpretation: The structure of the given compound and the structures of its two isomers containing four chlorine atoms are to be drawn.
Concept introduction: An isomer is defined as molecules having a same molecular formula but with a different structural arrangement of atoms within the formula. They do not have similar properties and functional group is also differing in their structures. Polychlorinated dibenzo-p-dioxins are a group of polyhalogenated organic compounds which are highly toxic substances released as a byproduct of chemical manufacturing processes.
To determine: The structure of the given compound and the structures of its two isomers containing four chlorine atoms.
Answer to Problem 113AE
Answer
The structure of the given compound
Explanation of Solution
Explanation
The structure of the given compound
The structure of the compound
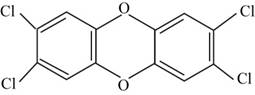
Figure 1
The structure of the isomer of
The structure of isomer
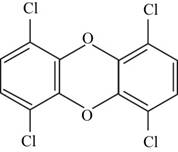
Figure 2
The chemical formula of the given compound is
The structure of the isomer of
The structure of isomer
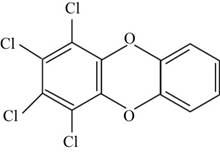
Figure 3
The chemical formula of the given compound is
Conclusion
The structure of the given compound
Want to see more full solutions like this?
Chapter 21 Solutions
Chemistry: An Atoms First Approach
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning





