
Organic Chemistry Plus Masteringchemistry With Pearson Etext, Global Edition
9th Edition
ISBN: 9781292151229
Author: Wade, LeRoy G.
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 21, Problem 21.45SP
Predict the products of the following reactions.
- a. phenol + acetic anhydride
- b. phenol + acetic formic anhydride
- c. aniline + phthalic anhydride
- d. anisole + succinic anhydride and aluminum chloride
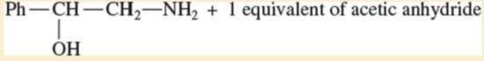
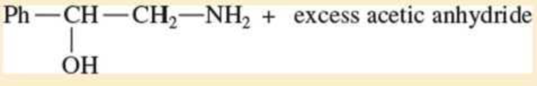
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
2) Another method for the above reaction starts with 4-aminophenol hydrochloride (the conjugate acid of 4-aminophenol). The treatment of this the hydrochloride salt with sodium ethanoate (acetate) buffer produces 4-aminophenol which is then able to react with ethanoic anhydride as per our method.
i. Why is 4-aminophenol hydrochloride not suitable for direct reaction with ethanoic anhydride?
ii. Draw a mechanism (i.e. curly arrows) showing the deprotonation of 4-aminophenol hydrochloride by sodium ethanoate to form 4-aminophenol (the free base).
(a) Account for the following :(i) Cl – CH2COOH is a stronger acid than CH3COOH.(ii) Carboxylic acids do not give reactions of carbonyl group.(b) Write the chemical equations to illustrate the following name reactions:(i) Rosenmund reduction (ii) Cannizzaro’s reaction(c) Out of CH3CH2 – CO – CH2 – CH3 and CH3CH2 – CH2 – CO – CH3, which gives iodoform test?
One possible side-reaction of this experiment is biphenyl, explain how it can be formed. Here is the oringial reaction
bromobenzene ----->magnesium. product is benze ring with MgBr attached to ring
Chapter 21 Solutions
Organic Chemistry Plus Masteringchemistry With Pearson Etext, Global Edition
Ch. 21.2F - Name the following carboxylic acid derivatives,...Ch. 21.4A - Prob. 21.2PCh. 21.4A - Prob. 21.3PCh. 21.4A - Prob. 21.4PCh. 21.5C - Prob. 21.7PCh. 21.6 - When ethyl 4-hydroxybutyrate is heated in the...Ch. 21.6 - Propose a mechanism for the following ring-opening...Ch. 21.6 - Prob. 21.15PCh. 21.7B - Prob. 21.16PCh. 21.7C - Prob. 21.19P
Ch. 21.7C - Prob. 21.20PCh. 21.7C - Prob. 21.21PCh. 21.7D - Prob. 21.22PCh. 21.7D - The mechanism for acidic hydrolysis of a nitrile...Ch. 21.8A - Prob. 21.24PCh. 21.8C - Prob. 21.25PCh. 21.9 - Prob. 21.26PCh. 21.9 - Prob. 21.27PCh. 21.9 - Prob. 21.28PCh. 21.10 - Draw a mechanism for the acylation of anisole by...Ch. 21.10 - Prob. 21.30PCh. 21.11 - Prob. 21.31PCh. 21.11 - Prob. 21.32PCh. 21.12 - Problem 21-33 Propose a mechanism for the...Ch. 21.12 - Suggest the most appropriate reagent for each...Ch. 21.12 - Show how you would synthesize each compound,...Ch. 21.13 - Prob. 21.36PCh. 21.13 - Prob. 21.37PCh. 21.14 - Prob. 21.38PCh. 21.14 - Prob. 21.39PCh. 21.16 - Prob. 21.40PCh. 21.16 - Prob. 21.41PCh. 21 - Prob. 21.42SPCh. 21 - Give appropriate names for the following...Ch. 21 - Predict the major products formed when benzoyl...Ch. 21 - Predict the products of the following reactions....Ch. 21 - Prob. 21.46SPCh. 21 - Prob. 21.47SPCh. 21 - Prob. 21.48SPCh. 21 - Propose mechanisms for the following reactions.Ch. 21 - Prob. 21.51SPCh. 21 - An ether extraction of nutmeg gives large...Ch. 21 - Prob. 21.53SPCh. 21 - Show how you would accomplish the following...Ch. 21 - Prob. 21.55SPCh. 21 - Prob. 21.56SPCh. 21 - Prob. 21.57SPCh. 21 - Prob. 21.58SPCh. 21 - Prob. 21.59SPCh. 21 - Explain this curious result. What does this...Ch. 21 - Prob. 21.61SPCh. 21 - Prob. 21.62SPCh. 21 - Prob. 21.63SPCh. 21 - A chemist was called to an abandoned aspirin...Ch. 21 - Prob. 21.67SPCh. 21 - The IR spectrum, 13ONTVTR spectrum, and 1HNMR...Ch. 21 - Prob. 21.69SPCh. 21 - Prob. 21.70SPCh. 21 - Prob. 21.71SP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Why do you wash the dichloromethane solution of your reductive amination product with sodium bicarbonate, rather than dilute aqueous HCl? a) Sodium bicarbonate is a good method of removing aldehydes from organic solvent.b) The amine product will be protonated by acid and remain in the aqueous layer as a salt.c) Sodium bicarbonate transfers the amine starting material into the aqueous layer.d) Sodium bicarbonate reacts with leftover NaBH(OAc)3 and removes it from the mixture.arrow_forwarda. Propose a mechanism for the formation of succinic anhydride in the presence of acetic anhydride.b. How does acetic anhydride make it easier to form the anhydride?arrow_forwardE. TESTS FOR PHENOLIC COMPOUNDSPhenolic compounds such as phenol, salicylic acid, etc., give characteristiccolors with FeCl3 and Millon’s Reagent.1. Ferric Chloride TestAdd 5 drops of FeCl3 solution to 4 different test tubes containing 1 mL each ofdilute solutions of the following compounds and note the color obtained.a. Phenol _______________________________________________________________b. Salicyclic acid ___________________________________________________________c. Resorcinol _______________________________________________________________d. Picric acid _______________________________________________________________2. Millon’s TestPrepare another set of 4 test tubes containing 1 mL each of the solutions listedbelow. Add 3 drops of Millon’s reagent to each and place all the test tubes in aboiling water bath. Note the color formed.a. Phenol _______________________________________________________________b. Salicylic acid ___________________________________________________________c.…arrow_forward
- (a) How will you carry out the following conversions?(i) Acetylene to Acetic acid (ii) Toluene to m-nitrobenzoic acid(iii) Ethanol to Acetone(b) Give reasons :(i) Chloroacetic acid is stronger than acetic acid.(ii) pH of reaction should be carefully controlled while preparing ammonia derivatives of carbonyl compounds.arrow_forward(a) Illustrate the following name reactions :(i) Hell-Volhard-Zelinsky reaction (ii) Wolff-Kishner reduction reaction(b) How are the following conversions carried out:(i) Ethylcyanide to ethanoic acid (ii) Butan-l-ol to butanoic acid(iii) Methylbenzene to benzoic acidWrite chemical equations for the involved reactions.arrow_forwardShow the product formed as a result of the reaction between propanoic acid and benzylalcohol in an acidic environment by writing down the reaction mechanism.arrow_forward
- An unknown compound is treated with peroxyacetic acid in dilute sulfuric acid. The product of reaction 1 is reacted with excess PBr3 , then excess NaNH2 (reaction 3). The product of reaction 3 is treated with lithium, then n-butylbromide (reaction 4). In reaction 5 the product of reaction 4 is reacted with ozone, then NaHSO3. This final reaction produced 2 acids, propanoic acid and pentanoic acid. What was the original unknown compouond?arrow_forwardWhat methods introduce the functional groups in the product ?arrow_forwardIn this study, the researcher compared S N 2 and E2reaction rates for four substrates. Three of the substrates had a second halogen on the bposition in the molecule. This work also compared the behavior of two nucleophiles:dianion I and II. You should read the abstract and look at Scheme 1 (p. 3082) and Table 4(p. 3086). Abstract: The gas-phase reactions of benzoate and phenolate containing dianions with a series of ‚-substitutedalkyl bromides (X-CH2CH2Br, X ) H, F, Cl, Br) have been studied in a quadrupole ion trap mass spectrometer.Branching ratios between SN 2 and E2 products were measured and rate constants were determined. The‚-halogens increase both the S N 2 and E2 rates, but the effect is greater for the latter process and thereforethese substituents lead to an increase in the amount of elimination. The kinetic data for the SN 2 reactions canbe analyzed via a two-parameter, linear free-energy relationship and the results indicate that field-effects (i.e.,electron-withdrawing…arrow_forward
- In this study, the researcher compared S N 2 and E2reaction rates for four substrates. Three of the substrates had a second halogen on the bposition in the molecule. This work also compared the behavior of two nucleophiles:dianion I and II. You should read the abstract and look at Scheme 1 (p. 3082) and Table 4(p. 3086). Abstract: The gas-phase reactions of benzoate and phenolate containing dianions with a series of ‚-substitutedalkyl bromides (X-CH2CH2Br, X ) H, F, Cl, Br) have been studied in a quadrupole ion trap mass spectrometer.Branching ratios between SN 2 and E2 products were measured and rate constants were determined. The‚-halogens increase both the S N 2 and E2 rates, but the effect is greater for the latter process and thereforethese substituents lead to an increase in the amount of elimination. The kinetic data for the SN 2 reactions canbe analyzed via a two-parameter, linear free-energy relationship and the results indicate that field-effects (i.e.,electron-withdrawing…arrow_forwardIn this study, the researcher compared S N 2 and E2reaction rates for four substrates. Three of the substrates had a second halogen on the bposition in the molecule. This work also compared the behavior of two nucleophiles:dianion I and II. You should read the abstract and look at Scheme 1 (p. 3082) and Table 4(p. 3086). Abstract: The gas-phase reactions of benzoate and phenolate containing dianions with a series of ‚-substitutedalkyl bromides (X-CH2CH2Br, X ) H, F, Cl, Br) have been studied in a quadrupole ion trap mass spectrometer.Branching ratios between SN 2 and E2 products were measured and rate constants were determined. The‚-halogens increase both the S N 2 and E2 rates, but the effect is greater for the latter process and thereforethese substituents lead to an increase in the amount of elimination. The kinetic data for the SN 2 reactions canbe analyzed via a two-parameter, linear free-energy relationship and the results indicate that field-effects (i.e.,electron-withdrawing…arrow_forward6 Please provide the correct answers. Thanks in advancearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:9781305577190
Author:Kenneth L. Williamson, Katherine M. Masters
Publisher:Brooks Cole
Lipids - Fatty Acids, Triglycerides, Phospholipids, Terpenes, Waxes, Eicosanoids; Author: The Organic Chemistry Tutor;https://www.youtube.com/watch?v=7dmoH5dAvpY;License: Standard YouTube License, CC-BY