
Organic Chemistry (9th Edition)
9th Edition
ISBN: 9780321971371
Author: Leroy G. Wade, Jan W. Simek
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 21.2F, Problem 21.1P
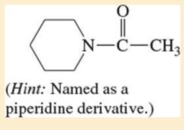
Name the following carboxylic acid derivatives, giving both a common name and an IUPAC name where possible.
- a. PhCOOCH2CH(CH3)2
- b. PhOCHO
- c. PhCH(CH3)COOCH3
- d. PhNHCOCH2CH(CH3)2
- e. CH3CONHCH2Ph
- f. CH3CH(OH)CH2CN
- g. (CH3)2CHCH2COBr
- h. Cl2CHCOCl
- i. (CH3)2CHCOOCHO
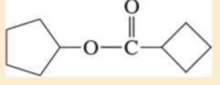
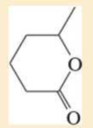
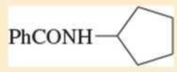
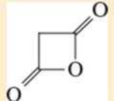
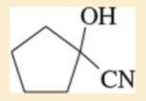
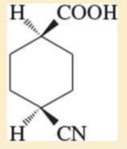
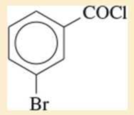
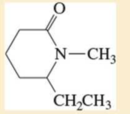
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
An acid chloride is more reactive than a carboxylic acid. But which reagent can convert a carboxylic acid into an acid chloride?
C6H5SO2Cl
PCC
Cl2/water
SOCl2
Which of the following carboxylic acids has the highest solubility in water?
A) CH₃CH₂C(=O)OH
B) CH₃CH₂CH₂CH₂C(=O)OH
C) CH₃(CH₂)₅C(=O)OH
D) CH₃(CH₂)₁₀C(=O)OH
E) All of these have equal solubility.
Which of the following substances will form Cr3+ ions upon reaction with Cr2O7(2-)?
a) 2-Butanol and But-2-eonic acid
b) 2-Butanol and Butanal
c) 2-Butanone and But-2-enoic acid
d) But-2-enoic acid and Butanal
e) Butanoic acid and 2-Butanol
Chapter 21 Solutions
Organic Chemistry (9th Edition)
Ch. 21.2F - Name the following carboxylic acid derivatives,...Ch. 21.4A - Prob. 21.2PCh. 21.4A - Prob. 21.3PCh. 21.4A - Prob. 21.4PCh. 21.5C - Prob. 21.7PCh. 21.6 - When ethyl 4-hydroxybutyrate is heated in the...Ch. 21.6 - Propose a mechanism for the following ring-opening...Ch. 21.6 - Prob. 21.15PCh. 21.7B - Prob. 21.16PCh. 21.7C - Prob. 21.19P
Ch. 21.7C - Prob. 21.20PCh. 21.7C - Prob. 21.21PCh. 21.7D - Prob. 21.22PCh. 21.7D - The mechanism for acidic hydrolysis of a nitrile...Ch. 21.8A - Prob. 21.24PCh. 21.8C - Prob. 21.25PCh. 21.9 - Prob. 21.26PCh. 21.9 - Prob. 21.27PCh. 21.9 - Prob. 21.28PCh. 21.10 - Draw a mechanism for the acylation of anisole by...Ch. 21.10 - Prob. 21.30PCh. 21.11 - Prob. 21.31PCh. 21.11 - Prob. 21.32PCh. 21.12 - Problem 21-33 Propose a mechanism for the...Ch. 21.12 - Suggest the most appropriate reagent for each...Ch. 21.12 - Show how you would synthesize each compound,...Ch. 21.13 - Prob. 21.36PCh. 21.13 - Prob. 21.37PCh. 21.14 - Prob. 21.38PCh. 21.14 - Prob. 21.39PCh. 21.16 - Prob. 21.40PCh. 21.16 - Prob. 21.41PCh. 21 - Prob. 21.42SPCh. 21 - Give appropriate names for the following...Ch. 21 - Predict the major products formed when benzoyl...Ch. 21 - Predict the products of the following reactions....Ch. 21 - Prob. 21.46SPCh. 21 - Prob. 21.47SPCh. 21 - Prob. 21.48SPCh. 21 - Propose mechanisms for the following reactions.Ch. 21 - Prob. 21.51SPCh. 21 - An ether extraction of nutmeg gives large...Ch. 21 - Prob. 21.53SPCh. 21 - Show how you would accomplish the following...Ch. 21 - Prob. 21.55SPCh. 21 - Prob. 21.56SPCh. 21 - Prob. 21.57SPCh. 21 - Prob. 21.58SPCh. 21 - Prob. 21.59SPCh. 21 - Explain this curious result. What does this...Ch. 21 - Prob. 21.61SPCh. 21 - Prob. 21.62SPCh. 21 - Prob. 21.63SPCh. 21 - A chemist was called to an abandoned aspirin...Ch. 21 - Prob. 21.67SPCh. 21 - The IR spectrum, 13ONTVTR spectrum, and 1HNMR...Ch. 21 - Prob. 21.69SPCh. 21 - Prob. 21.70SPCh. 21 - Prob. 21.71SP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Give reasons: (i) Bond length of C = O in carboxylic acids is slightly larger than C = O bond length in carbonyl compounds. (ii) There are two –NH2 groups in semicarbazide. However, only one –NH2 group is involved in the formation of semicarbazones. (iii) Benzoic acid is less soluble in water than acetic acid. (iv) Formic acid is a stronger acid than acetic acid.arrow_forwardList the following compounds in order of increasing water solubility: a.ethoxyethane b.propanoic acid c.pentane d.1 butanolarrow_forward(a) Give chemical tests to distinguish between the following pairs of compounds :(i) Pentan-2-ol and Pentan-3-ol (ii) Methanol and Phenol(b) o-nitro phenol is more acidic than o-methoxy phenol. Explain why.arrow_forward
- Which of the following carboxylic acid is toxic when ingested? a.Citric acid b.Malic acid c.Acetic acid d.Oxalic acid What is the general formula for Grignard reagents? a.RCOOH b.RMgX c.RX d.RCOX Oxidation of alkyl benzenes in the presence of Sulfuric acid will produce which carboxylic acid? a.Cyclohexanecarboxylic acid b.Benzoic acid c.Acrylic acid d.Fumaric acid What dicarboxylic acid contains six carbon atoms in its structure? a.Capric acid b.Pimelic acid c.Adipic acid d.Hexanoic acid Which of the following is an example of an unsaturated carboxylic acid? a.Succinic acid b.Formic acid c.Glycolic acid d.Acrylic acidarrow_forwardShow how to prepare pentanoic acid from each component. (a) 1-Pentanol (b) Pentanal (c) 1-Pentene (d) 1-Butanol (e) 1-Bromopropane (f) 1-Hexenearrow_forwardComplete the reaction predicting the products: Name all functional groups for reactants/Products , Sketch/Name all reactants/Products using IUPAC rules, and determine if each reaction is Reversible or Non-Reversible. Classify product if possible as a ketal/acetal/hemi-ketal/hemi- acetal a. e. b. acid chloride and alcohol (non-reversible) Ester with halogen Propyl 2-chlorobutanoate c. heptanoic acid ,+ ethanol larr→ d. butyl butanoate +NaOH→ e. hexyl ethanoate +NaOH→ N-011 uster strong base (non-reversible) (Or 1-hydroxyhexane) i need help with A,C, D,E..arrow_forward
- Why are carbonyl compounds considered weakly acidic? Would you expect carbonyl compounds to be more acidic than alkanes? Explain.arrow_forward1. O-hydroxybenzoic acid is a major product formed with phenol and which other reactant/s I-primary alcohol II-sodium hydroxide III-water IV-carbon dioxide A.I and III B. I and IV C. II and III D. II and IVarrow_forwardWhich carboxylic acid listed below is the most water soluble? 2-chlorobenzoic acid acetic acid octanoic acid 2,3-dimethylhexanoic acidarrow_forward
- 1. Cite some reactions in which formaldehyde behaves differently from other aldehydes. 2. Give some biological and medical applications of: a. formalin b. chloral c. urotropinearrow_forwarda. Rank the following carboxylic acids from strongest to weakest acid: CH3CH2CH2COOH CH3CH2CHCOOH ClCH2CH2CH2COOH CH3CHCH2COOH b. How does the presence of an electronegative substituent such as Cl affect the acidity of a carboxylic acid? c. How does the location of the substituent affect the acidity of the carboxylic acid?arrow_forwardGive the products formed when benzaldehyde and benzoic acid are treated with the given reagents. a. Tollen’s reagentb. phenylhydrazine, H+c. HCNd. NH2OHe. 1 mole H2, Nif. 1 mole CH3OH, H+g. LiAlH4 then H2O, H+h. 2 moles CH3OH, H+i. CH3MgCl, then H2O, H+j. H2Oarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
Nomenclature: Crash Course Chemistry #44; Author: CrashCourse;https://www.youtube.com/watch?v=U7wavimfNFE;License: Standard YouTube License, CC-BY