
Interpretation:
The conversion of D-galactose to D-galacturonic acid is to be suggested, by carrying out specific oxidation.
Concept introduction:
舧 A carbohydrate is a
舧
舧 Carbohydrates are oxidized by
舧 Aldaric acids are carbohydrates having two carboxylic acids. They are formed due to oxidation reaction of aldoses with dilute
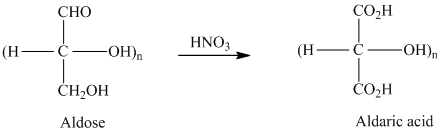
舧 The molecules that are nonsuperimposable or not identical with their mirror images are known as chiral molecules.
舧 A pair of two mirror images that are nonidentical is known as enantiomers, which are optically active.
舧 The stereoisomers that are nonsuperimposable on each other and not mirror images of each other are known as diastereomers.
舧 The achiral compounds in which plane of symmetry is present internally and consists of chiral centres are known as meso compounds, but they are optically inactive.
舧 Compounds that have a plane of symmetry tend to exist in meso forms. A meso form arises when the two stereoisomers produce superimposable images, and hence, compounds having meso forms are optically inactive.
舧 The reaction in which there is removal of water molecule is called dehydration reaction.
舧
舧 Monosaccharide carbohydrates that have their
舧 Based on the given information, direct oxidation of an aldose affects its aldehyde group first, converting it to a carboxylic acid. Most oxidizing agents that attack
Want to see the full answer?
Check out a sample textbook solution
Chapter 22 Solutions
EBK ORGANIC CHEMISTRY
- What is the product of the starting material D-glyceraldehyde which will (1) produce aldaric acid upon reacting with HNO3 + H2O, NaOCH2, NH2OH, and (CH3CO)2O + NaOCOCH3 (2) produce tartaric acid upon reacting with HNO3 + H2O, NaOCH3, NH2OH, and (CH3CO)2O + NaOCOCH3arrow_forwardThe cyclic hemiacetal is more stable than the open-chain form, so very little of the open-chain form is present atequilibrium. Will an aqueous solution of glucose reduce Tollens reagent and give a positive Tollens test? Explain.arrow_forwardd-Glucuronic acid is found widely in plants and animals. One of its functions is to detoxify poisonous HO-containing compounds by reacting with them in the liver to form glucuronides. Glucuronides are water soluble and, therefore, readily excreted. After ingestion of a poison such as turpentine or phenol, the glucuronides of these compounds are found in urine. Draw the structure of the glucuronide formed by the reaction of beta-d-glucuronic acid and phenol.arrow_forward
- 25.1a) Predict the product of the following reactions 1) - MgBr 2) H,0 b) -MgBr 2) Ноarrow_forward2. In the synthesis of peptides, carboxylic acids are condensed with amines in the present of a reagent such as dicyclohexylcarbodiimide (DCC) [Section 25.6]. a. Propose a mechanism for the following, using curved arrow notation. H₂N. glycine OH H₂N 요. glycine OH DCC H₂N. N H gly-gly LOHarrow_forwardExplain briefly why sulfur mustard is soluble in the lipid bilayer membranes that surround human cells and in many fatty hydrocarbon polymers, while thiodiglycol used to synthesize sulfur mustard is less soluble in these hydrophobic materials and has greater solubility in water?arrow_forward
- 22.47 Tertiary amines with three different alkyl groups are chiral but cannot be resolved because pyramidal inversion causes racemization at room temperature. Nevertheless, chiral aziridines can be resolved and stored at room temperature. Aziridine is a three-membered heterocycle containing a nitrogen atom. The following is an example of a chiral aziridine. In this compound, the nitrogen atom is a chiral center. Suggest a reason why chiral aziridines do not undergo racemization at room temperature.arrow_forwarda) Write and name of the mechanlam when chloroethane is refluxed with ethenolic polassium cyanide.arrow_forwardDraw the structure of each of the following molecules. (a) 5-phenylpentanamide; (b) (2S,3S)-2,3-dimethoxyhexanediamide; (c) N-phenylcyclobutanecarboxamidearrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning



