
(a)
Interpretation:
The synthesis is to be shown for the 4-chloro-3-nitrobenzoic acid from toluene.
Concept introduction:
Nitration: The formation of nitro group in a
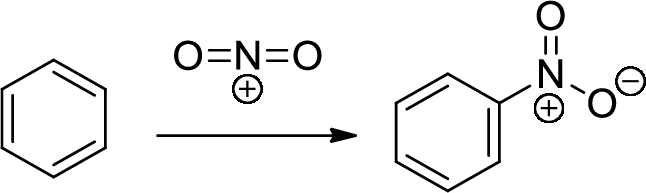
Chromic Acid:
Chromic Acid (
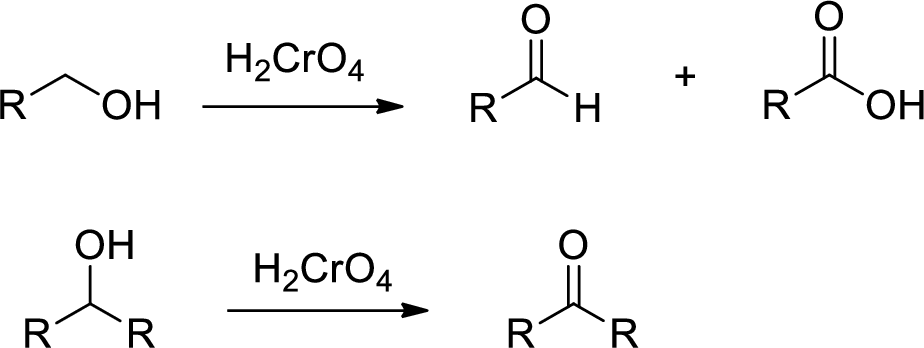
(b)
Interpretation:
The reagent and condition is to be proposed for step 1.
(c)
Interpretation:
The mechanism is to be proposed for step 2.
Concept introduction:
Ipso substitution reaction: It is the one of the
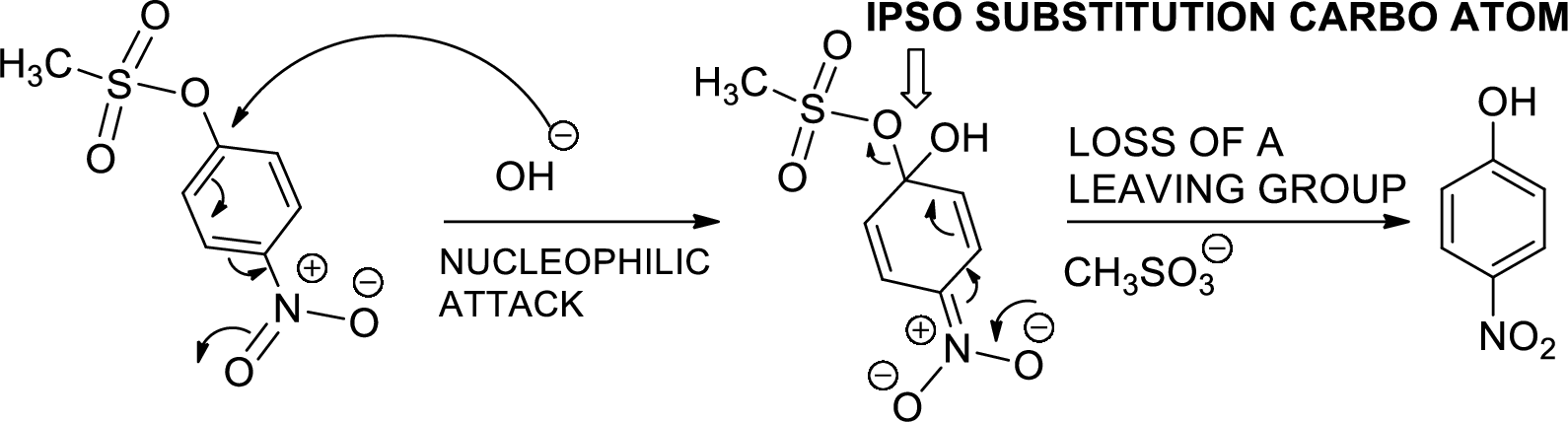
(d)
Interpretation:
The reagent and condition is to be proposed for step 3.
Concept introduction:
Hydrogenolysis:
Metal catalyst gives the corresponding amine or alcohol.
Reductive amination reaction: Amination is the process by which an amine group is introduced into an organic molecule.
The conversion of Carbonyl group in to amine via imine intermediate is called reductive amination.
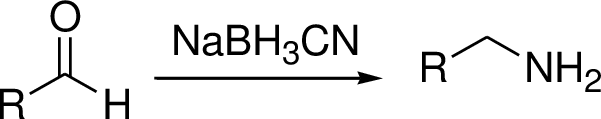
(e)
Interpretation:
The possible stereoisomer’s has to be shown if the product is chiral.
Concept introduction:
Isomer: A molecule having the same molecular formula but with different chemical structure is called isomer.
Enantiomers: A compound which is non-superimposable mirror image is called enantiomers.
Diastereomers: A compound which is non-superimposable and non-mirror image is called diastereomers.
Racemic mixture: A racemic mixture is simply a mixture containing an equal amount of each enantiomer.
Achiral:
A molecule is superimposable on its mirror image is called achiral molecule.
Trending nowThis is a popular solution!

Chapter 23 Solutions
Organic Chemistry
- Please don't provide handwritten solution ..... Propose a reasonable reaction mechanism for the transformation given below and provide a brief explanationarrow_forwardTestosterone is one of the most important male steroid hormones. When testosterone is hydrated by treatment with acid, rearrangement occurs to yield the product shown. Propose a mechanism to account for this reaction.arrow_forwardPredict the products, propose mechanisms for the reactions of carboxylic acidderivatives with reducing agents, alcohols, amines, and organometallic reagentssuch as Grignard, organolithium, and organocuprate reagentsarrow_forward
- Predict the structures and show the mechanism of formation of compounds A and B.You can skip the mechanism of ester hydrolysis.arrow_forwardSuggest possible synthetic route for each of following transformations. Show the reagents, intermediates and products.arrow_forwardSuggest possible synthetic route for each of following transformations. Show the reagents,intermediates and products.arrow_forward
- Synthesize the following product via acetoacetic ester synthesis. Use any necessary reagents. Show the mechanisms involved.arrow_forwardSuggest the reagents for the synthesis of the following compoundsarrow_forwardShow how you would synthesize the following compounds, starting with benzene or toluene and any necessary acyclicreagents. Assume para is the major product (and separable from ortho) in ortho, para mixtures. p-aminobenzoic acidarrow_forward
- Provide a detailed mechanism for the following (Hint:halohydrin reaction). Use arrows to show electron flow and show intermediates.arrow_forwardFor the following reaction scheme, identify by drawing the reagents b and d and the intermediate c that are formed in the synthesis of benzoic acid.arrow_forwardWhen 1,4-dibromobutane was treated with excess ethanamine at propanone, the main product had the molecular formula C6H13N. Deduce the structure of this product by proposing a mechanism for the reaction.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
