
Concept explainers
(a)
Interpretation:
Synthesis of benzylamine from the given starting material has to be shown.
Concept Introduction:
Preparation of imine:
An imine is a compound having
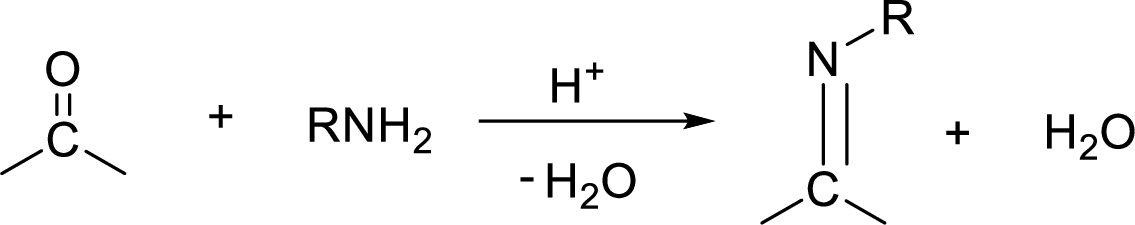
Reduction: If electrons are gained to a species or hydrogen atoms are added to a species or oxygen atom gets removed from a species during a
(b)
Interpretation:
Synthesis of benzylamine from the given starting material has to be shown.
Concept Introduction:
Amide Hydrolysis: In presence of base, amide reacts with water to form the corresponding amine and carboxylic acid salt.
(c)
Interpretation:
Synthesis of benzylamine from the given starting material has to be shown.
Concept Introduction:
Preparation of amine: A primary amine is formed when an
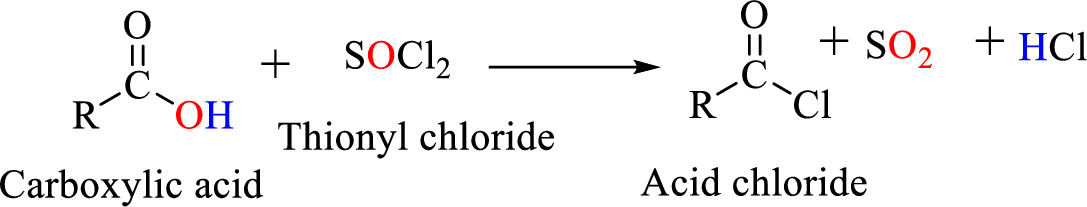
Thionyl chloride:
(d)
Interpretation:
Synthesis of benzylamine from the given starting material has to be shown.
Concept Introduction:
Thionyl chloride:
(e)
Interpretation:
Synthesis of benzylamine from the given starting material has to be shown.
Concept Introduction:
Acid chlorides are most often prepared by treating a carboxylic acid with thionyl chloride.
Amide Formation: Amide is formed when an acid chloride reacts with an amine or ammonia.
Here, the chlorine atom that is attached to the carbonyl carbon atom of the acid chloride is being replaced by
Reduction: If electrons are gained to a species or hydrogen atoms are added to a species or oxygen atom gets removed from a species during a chemical reaction is known as reduction. In a reaction,
(f)
Interpretation:
Synthesis of benzylamine from the given starting material has to be shown.
Concept Introduction:
Preparation of amide: An amide is formed when an ester is reacted with ammonia.
Reduction: If electrons are gained to a species or hydrogen atoms are added to a species or oxygen atom gets removed from a species during a chemical reaction is known as reduction. In a reaction,
Trending nowThis is a popular solution!

Chapter 23 Solutions
Organic Chemistry
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

