
(a)
Interpretation:
Amino acid from Table 23.1 should be classified into each of the categories nonpolar v. polar.
Concept introduction:
Proteins are
They polymerize by peptide linkage to form dipeptide, oligopeptide and polypeptide molecules. Each peptide bond formation is a condensation reaction that occurs with the elimination of water molecule.
Answer to Problem 6SSC
Glycine, valine and phenylalanine are nonpolar amino acids and, serine, cysteine, lysine, glutamic acid, glutamine are polar amino acids.
Explanation of Solution
Polar amino acid is directed towards the outside of the protein because of the hydrophilic properties of side chain whereas the nonpolar amino acid is directed towards the inside of protein (R group repelled by water) because of the hydrophobic nature.
The given amino acids are: Glycine, Serine, Cysteine, Lysine, Glutamic acid, Glutamine, Valine and Phenylalanine.
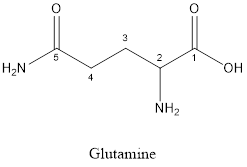
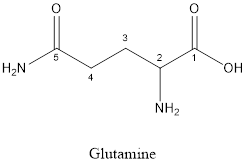
The structure of Glutamine is:
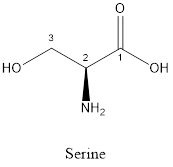
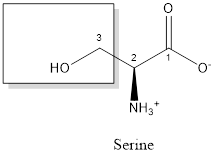
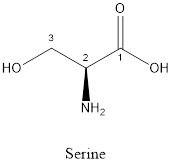
The structure of serine is:
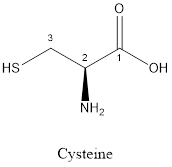
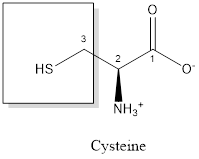
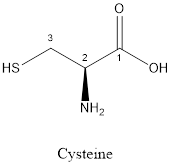
The structure of Cysteine is:
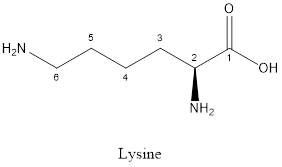
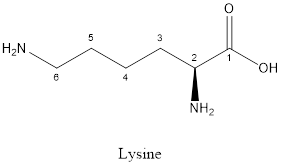
The structure of Lysine:
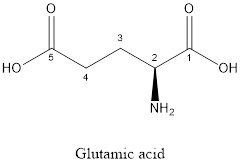
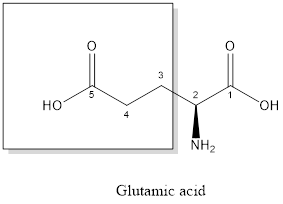
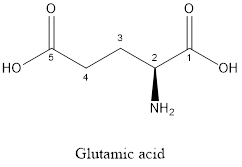
The structure of glutamic acid:
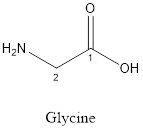
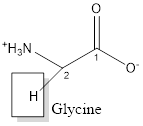
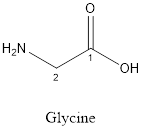
The structure of Glycine is:
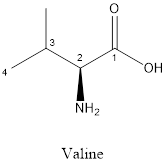
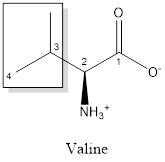
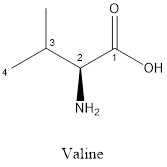
The structure of valine is:
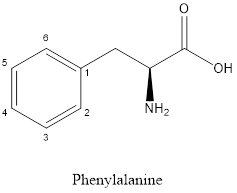
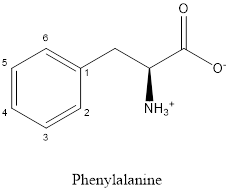
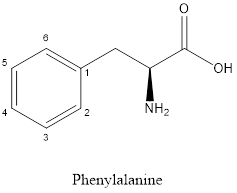
The structure of phenylalanine is:
Glycine is nonpolar as it is neutral species; also nonpolar hydrogen is linked as shown below:
Valine is nonpolar as it is neutral species; also nonpolar group is linked as shown below:
Serine is polar amino acid as it consists of polar R group:
Cysteine is polar amino acid as it consists of polar R group:
Glutamine is a polar amino acid as it consists of polar R group:
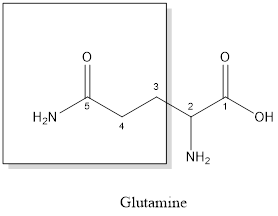
Lysine is a polar amino acid as it consists of polar R group:
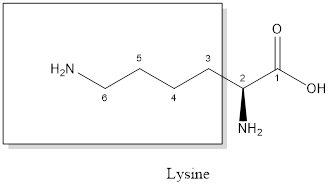
Glutamic acid is a polar amino acid as it consists of polar R group:
Phenylalanine is nonpolar as it is neutral species; also nonpolar group is linked as shown below:
Therefore; glycine, valine and phenylalanine are nonpolar amino acids and, serine, cysteine, lysine, glutamic acid, glutamine are polar amino acids.
(b)
Interpretation:
Amino acid from Table 23.1 should be classified into each of the categories aromatic v. aliphatic.
Concept introduction:
Proteins are polymeric biomolecules which are formed by the polymerisation of amino acids. Amino acids are the organic molecules with
They polymerize by peptide linkage to form dipeptide, oligopeptide and polypeptide molecules. Each peptide bond formation is a condensation reaction that occurs with the elimination of water molecule.
Answer to Problem 6SSC
The amino acids which are aliphatic in nature are glycine, serine, cysteine, lysine, glutamic acid, glutamine, valine whereas the amino acid which is aromatic.
Explanation of Solution
A cyclic ring structure which contains carbon atoms and hydrogen atoms is known as aromatic ring and the compound which has aromatic ring is known as
The compound which consists of carbon and hydrogen linked with each other either in a straight chain, branched chain and non-aromatic rings is known as aliphatic compounds.
The given amino acids are: Glycine, Serine, Cysteine, Lysine, Glutamic acid, Glutamine, Valine and Phenylalanine.
The structure of Glutamine is:
The structure of serine is:
The structure of Cysteine is:
The structure of Lysine:
The structure of glutamic acid:
The structure of Glycine is:
The structure of valine is:
The structure of phenylalanine is:
According to above structures, the amino acids which are aliphatic in nature are glycine, serine, cysteine, lysine, glutamic acid, glutamine, valine as these amino acids contain carbon and hydrogen bonds (hydrocarbon) whereas the amino acid which is aromatic is phenylalanine as it contains aromatic ring in its side chain.
(c)
Interpretation:
Amino acid from Table 23.1 should be classified into each of the categories acidic v. basic.
Concept introduction:
Proteins are polymeric biomolecules which are formed by the polymerisation of amino acids. Amino acids are the organic molecules with
They polymerize by peptide linkage to form dipeptide, oligopeptide and polypeptide molecules. Each peptide bond formation is a condensation reaction that occurs with the elimination of water molecule.
Answer to Problem 6SSC
Lysine and Glutamic acid is basic and acidic amino acid. The remaining amino acids are neutral.
Explanation of Solution
The amino acids which contain R group as a carboxylic acid group is known as acidic amino acid.
The amino acids which contain R group as a
The given amino acids are: Glycine, Serine, Cysteine, Lysine, Glutamic acid, Glutamine, Valine and Phenylalanine.
The structure of Glutamine is:
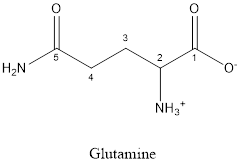
Glutamine is neither acidic nor basic as the side chain of glutamine is uncharged (polar R group). Thus, glutamine is a neutral amino acid.
The structure of serine is:
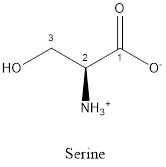
Serine is neither acidic nor basic as the side chain of serine is uncharged (polar R group). Thus, serine is a neutral amino acid.
The structure of Cysteine is:
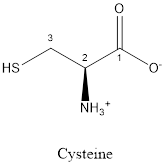
Cysteine is neither acidic nor basic as the side chain of cysteine is uncharged (polar R group). Thus, cysteine is a neutral amino acid.
The structure of Lysine:
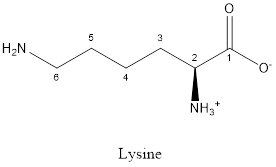
Lysine is basic in nature as the side chain of lysine consists of amine group. Thus, lysine is a basic amino acid.
The structure of glutamic acid:
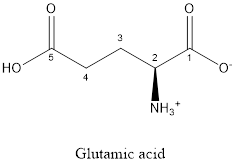
Glutamic acid is acidic in nature as the side chain of glutamic acid consists of carboxylic group. Thus, Glutamic acid is an acidic amino acid.
The structure of Glycine is:
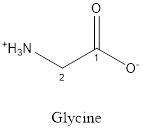
Glycine is neither acidic nor basic as the side chain of glycine is uncharged (polar R group). Thus, glycine is a neutral amino acid.
The structure of valine is:
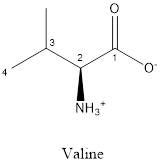
Valine is neither acidic nor basic as the side chain of valine is uncharged (polar R group). Thus, valine is a neutral amino acid.
The structure of phenylalanine is:
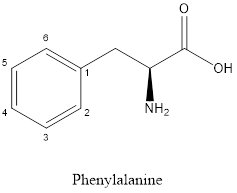
Phenylalanine is neither acidic nor basic as the side chain of phenylalanine is uncharged (polar R group). Thus, phenylalanine is a neutral amino acid.
Chapter 23 Solutions
Chemistry: Matter and Change
Additional Science Textbook Solutions
Essential Organic Chemistry (3rd Edition)
Organic Chemistry (9th Edition)
Organic Chemistry
Chemistry: The Central Science (14th Edition)
Chemistry: A Molecular Approach
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





