
Interpretation:
The number of possible isomers for galactose, glucose and fructose should be calculated.
Concept introduction:
The compounds having similar chemical formula but different structures are known as isomers.
The compounds having similar chemical or molecular formula but different connectivity is known as constitutional isomers.
Number of stereoisomers is calculated by below expression:
Where, n = number of chiral carbons
Answer to Problem 10SSC
Number of isomers of glucose = 16
Number of isomers of galactose = 16
Number of isomers of fructose = 8
Explanation of Solution
Glucose consists of single unit of sugar which can’t be hydrolyzed into simpler molecule.
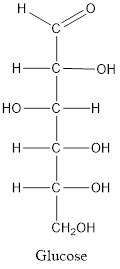
When a carbon atom is linked with different groups is known as chiral carbon and center is known as chiral center. Chiral center is represented by a symbol *.
The structure is drawn as with symbol *:
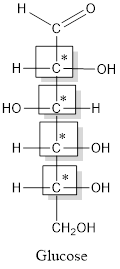
Thus, four chiral centers are present in the above structure as first four carbon atoms are linked with four different groups.
Number of isomers is calculated as:
Here, n = 4 (3 chiral carbon atoms are present)
Number of isomers =
= 16
Therefore, sixteen isomers are present for the structure of glucose.
Galactose consists of single unit of sugar which can’t be hydrolyzed into simpler molecule.
In galactose, the hydroxyl group linked with forth carbon atom of the chain is present on left side whereas in glucose, the hydroxyl group linked with forth carbon atom of the chain is present on right side.
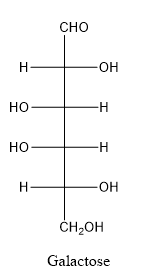
When a carbon atom is linked with different groups is known as chiral carbon and center is known as chiral center. Chiral center is represented by a symbol *.
The structure is drawn as with symbol *:
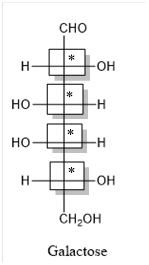
Thus, four chiral centers are present in the above structure as first four carbon atoms are linked with four different groups.
Number of isomers is calculated as:
Here, n = 4 (3 chiral carbon atoms are present)
Number of isomers =
= 16
Therefore, sixteen isomers are present for the structure of galactose.
Fructose consists of single unit of sugar which can’t be hydrolyzed into simpler molecule.
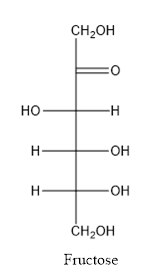
When a carbon atom is linked with different groups is known as chiral carbon and center is known as chiral center. Chiral center is represented by a symbol *.
The structure is drawn as with symbol *:
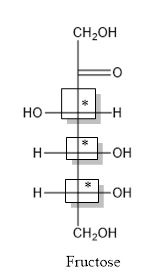
Thus, three chiral centers are present in the above structure as first three carbon atoms are linked with four different groups.
Number of isomers is calculated as:
Here, n = 3 (3 chiral carbon atoms are present)
Number of isomers =
= 8
Therefore, eight isomers are present for the structure of fructose.
Chapter 23 Solutions
Chemistry: Matter and Change
Additional Science Textbook Solutions
Organic Chemistry (9th Edition)
Chemistry: Structure and Properties
General Chemistry: Principles and Modern Applications (11th Edition)
Chemistry: The Central Science (13th Edition)
Essential Organic Chemistry (3rd Edition)
Organic Chemistry (8th Edition)
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





