
Concept explainers
(a) On the basis of the following sequence of reactions, Emil Fischer was able to show that
(b) The configuration of L-(+)-cysteine can be related to that of L-(−-)-serine through the following reactions. Write Fischer projections for D and E:
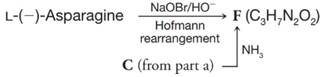
(c) The configuration of L-(−-)-asparagine can be related to that of L-(−-)-serine in the following way. What is the structure of F?
Want to see the full answer?
Check out a sample textbook solution
Chapter 24 Solutions
Organic Chemistry
Additional Science Textbook Solutions
Living By Chemistry: First Edition Textbook
Basic Chemistry (5th Edition)
Introductory Chemistry (6th Edition)
General Chemistry: Atoms First
Chemistry: A Molecular Approach
Chemistry & Chemical Reactivity
- Isoleucine and threonine are the only two amino acids with two chirality centers. Assign R or S configuration to the methyl-bearing carbon atom of isoleucine.arrow_forwardD-Glicose reacts with acetone in the presence of acid to yield the nonreducing 1, 2: 5, 6-diisopropylidene-D-glucofuranose. Propose a mechanism.arrow_forwardEvidence for the role of acetate in fatty-acid biosynthesis comes from isotopelabeling experiments. If acetate labeled with 13C in the methyl group (13CH3CO2H) were incorporated into fatty acids, at what positions in the fattyacid chain would you expect the 13C label to appear?arrow_forward
- Mannose is an aldohexose with relative stereochemistry AABB. i) Draw the Fischer projection of 2-amino-2-deoxy-D-mannose. ii) Construct the Haworth projection of 2-amino-2-deoxy-D-mannose. iii) Assign the absolute configuration for C-3 in 2-amino-2-deoxy-D-mannose.arrow_forwardThe 1H NMR spectrum of d-glucose in D2O exhibits two high-frequency doublets. What is responsible for these doublets?arrow_forwardOne of the steps in fatty-acid biosynthesis is the dehydration of (R)-3-hydroxybutyryl ACP to give trans-crotonyl ACP. Does the reaction remove the pro-R or the pro-S hydrogen from C2?arrow_forward
- Assign R or S configuration to each chirality center in the following molecular model of the amino acid isoleucine (blue = N):arrow_forward(R)-Pulegone is converted to (R)-citronellic acid by addition of HCl followed by treatment with NaOH. Propose a mechanism for each step in this transformation and account for the regioselectivity of HCl addition.arrow_forwardThe enzyme that catalyzes the Ca ¬ Cb bond cleavage reaction that converts serine to glycine removes thesubstituent (R) bonded to the a-carbon in the first step of the reaction. Starting with PLP bound to serine inan imine linkage, propose a mechanism for this reaction. (Hint: The first step involves removal of the protonfrom serine’s OH group.)arrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning


