
Organic Chemistry
12th Edition
ISBN: 9781118875766
Author: T. W. Graham Solomons, Craig B. Fryhle, Scott A. Snyder
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Question
Chapter 24, Problem 6PP
Interpretation Introduction
Interpretation:
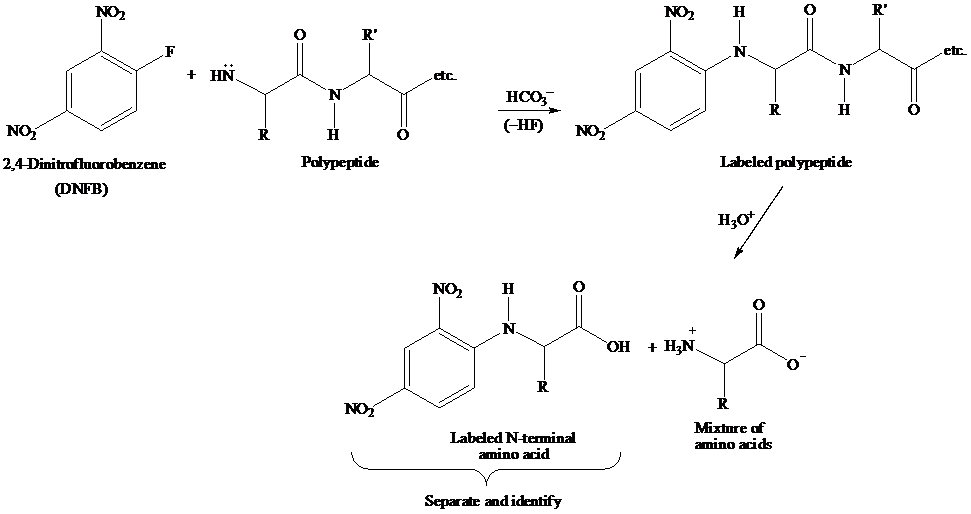
The reaction showing 2,4-dinitrofluorobenzene which could be used to identify the n-terminal amino acid of VAG is to be written. Further, the product of hydrolysis, when VKG is treated with 2,4-dinitrofluorobenzene, is to be identified.
Concept introduction:
The Sanger N-Terminal analysis is a method for sequence analysis of amino acid using 2,4-dinitrofluorobenzene (DNFB). The nucleophilic
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Show the peptides that would result from cleavage by the indicated reagent:
Val-Arg-Gly-Met-Arg-Ala-Ser by carboxypeptidase A
An unknown decapeptide was isolated and characterized. Complete hydrolysis of this peptide gave : F(2), A,G,C,K,N,T, W and V. Treatment with carboxypeptidase releases A. Reaction with Edman’s reagent gave PTH-T and a nonapeptide. The nonapeptide was treated with trypsin and gave 2 peptides: (V-C-G-A) and (N-FF-W-K). Give the sequence of amino acid in the decapeptide.
Write a structural formula for the product formed by treatment of the N-terminal amino group with Sanger’s reagent and propose a mechanism for its formation.
Chapter 24 Solutions
Organic Chemistry
Ch. 24 - Prob. 1PPCh. 24 - Practice Problem 24.2 The guanidino group NHNHCNH2...Ch. 24 - Prob. 3PPCh. 24 - Prob. 4PPCh. 24 - Prob. 5PPCh. 24 - Prob. 6PPCh. 24 - Prob. 7PPCh. 24 - Practice Problem 24.8
Glutathione is a tripeptide...Ch. 24 - Prob. 9PPCh. 24 - Prob. 10PP
Ch. 24 - Prob. 11PPCh. 24 - Practice Problem 24.12 Show all steps in the...Ch. 24 - Practice Problem 24.13 The synthesis of a...Ch. 24 - Practice Problem 24.14
The terminal carboxyl...Ch. 24 - Prob. 15PPCh. 24 - Prob. 16PPCh. 24 - (a) Which amino acids in Table 24.1 have more than...Ch. 24 - Prob. 18PCh. 24 - 24.19 (a) On the basis of the following sequence...Ch. 24 - Prob. 20PCh. 24 - Prob. 21PCh. 24 - Prob. 22PCh. 24 - Prob. 23PCh. 24 - Prob. 24PCh. 24 - The enzyme lysozyme and its mechanism are...Ch. 24 - Prob. 2LGP
Knowledge Booster
Similar questions
- Somatostatin is a tetradecapeptide of the hypothalamus that inhibits the release of pituitary growth hormone. Its amino acid sequence has been determined by a combination of Edman degradations and enzymic hydrolysis experiments. On the basis of the following data, deduce the primary structure of somatostatin: 1. Edman degradation gave PTH-Ala. 2. Selective hydrolysis gave peptides having the following indicated sequences: Phe-Trp Thr-Ser-Cys Lys-Thr-Phe Thr-Phe-Thr-Ser-Cys Asn-Phe-Phe-Trp-Lys Ala-Gly-Cys-Lys-Asn-Phe 3. Somatostatin has a disulfide bridge.arrow_forwardLeuprolide is a synthetic nonapeptide used to treat both endometriosis in women and prostate cancer in men. (a) Both C-terminal and N-terminal amino acids in leuprolide have been structurally modified. Identify the modifications. (b) One of the nine amino acids in leuprolide has d stereochemistry rather than the usual L. Which one? (c) Write the structure of leuprolide using both one- and three-letter abbreviations. (d) What charge would you expect leuprolide to have at neutral pH?arrow_forwardThe peptide Proline-Serine-Alanine-Phenylalanine-Glutamine is present at pH 7. Draw the peptide and include stereochemistry.arrow_forward
- The Arg-Gly-Asp tripeptide (RGD) is a well-known tumour targeting peptidemotif. Explain how you would synthesise H-Arg-Gly-Asp-OH, starting fromthe constituent amino acids? Explain all steps that are necessary and discuss amechanism for one side chain protection and one C terminus protection.arrow_forwardA nonapeptide released by globulins in the blood in response to a waspsting. Hydrolysis gives the following amino acids: 2 Arg, Gly, 2 Phe, 3 Pro, and Ser.Edman degradation gives phenylthiohydantoin of Arg. Cleavage with carboxypeptidase gives Arg. Partial hydrolysis gives the following di- and tripeptides: Phe-Ser, Pro-GlyPhe, Pro-Pro, Ser-Pro-Phe, Phe-Arg, and Arg-Pro. What is the amino acid sequence of this peptide?arrow_forwardBrucine is a poisonous alkaloid obtained from Strychnos nux vomica, a tree that grows in India, Sri Lanka, and northern Australia. Write out a resolution scheme, which shows how a racemic mixture of phenylalanine can be resolved using brucine.arrow_forward
- 4b) Canavanine is closely related to arginine, and like arginine its side group has a +1 charge when protonated. If you dissolved canavanine in an aqueous solution at pH 10, what would the net charge on a molecule of canavanine be? Please show your work or make it clear how you determined the charge contribution from each ionizable group.arrow_forwardAnother strategy used to resolve amino acids involves converting the carboxy group to an ester and then using a chiral carboxylic acid to carry out an acid–base reaction at the free amino group. Using a racemic mixture of alanine enantiomers and (R)-mandelic acid as resolving agent, write out the steps showing how a resolution process would occur.arrow_forwardGiven the following peptide SEPIMAPVEYPK(a) Estimate the net charge at pH 7 and at pH 12. Assume the pKa valuesgiven in as shown. (b) How many peptides would result if this peptide were treated with(1) cyanogen bromide, (2) trypsin, or (3) chymotrypsin?(c) Suggest a method for separating the peptides produced by chymotrypsintreatment.arrow_forward
- Given the following peptideSEPIMAPVEYPK(a) Estimate the net charge at pH 7 and at pH 12. Assume the pKa valuesgiven in Table (b) How many peptides would result if this peptide were treated with(1) cyanogen bromide, (2) trypsin, or (3) chymotrypsin?(c) Suggest a method for separating the peptides produced by chymotrypsintreatment.arrow_forwardSerine esterase contains a catalytic triad at its active site. Which amino acid in serine esterase is responsible for mediating general acid catalysis for the breakdown of tetrahedral intermediate to the carboxyl product? Explain.arrow_forwardA tripeptide on hydrolysis produced glycine, alanine and leucine. The structures of these amino acids are shown below. On reaction with Edman’s reagent, leucine was released as the phenylhydantoin. Treatment of the tripeptide with carboxypeptidase gave glycine. Draw the structure of the tripeptide.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
