
Interpretation:
The Strecker synthesis of DL-phenylalanine is to be outlined, and by preparing the starting
Concept introduction:
舧 Amino acids are organic compounds containing
舧 Strecker synthesis involves treating an aldehyde with ammonia and hydrogen cyanide to give an aminonitrile, which can be hydrolyzed to an αa-amino acid.
The general reaction is as:
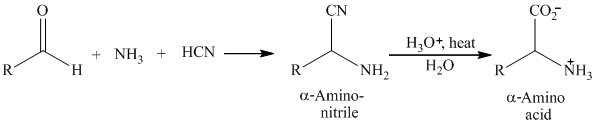
舧 The Strecker synthesis involves formation of imine from phenyl acetaldehyde and ammonia, which on addition of hydrogen cyanide produces
舧 Acrolein and methanethiol on treating with a base gives
Want to see the full answer?
Check out a sample textbook solution
Chapter 24 Solutions
Organic Chemistry
- Write out the steps needed to synthesize the following peptide using the Merrifield method.arrow_forwardIn a paragraph form, provide the experimental procedures with the laboratory equipments that needs to be used in the removal of carbon dioxide and Nucleophilic addition on the nitrogen of isoxazoline that is present in the mechanism of reaction of protein that contain native serine residues by the reaction of oxazetidine-containing peptides and α-keto acidarrow_forwardAn unknown decapeptide was isolated and characterized. Complete hydrolysis of this peptide gave : F(2), A,G,C,K,N,T, W and V. Treatment with carboxypeptidase releases A. Reaction with Edman’s reagent gave PTH-T and a nonapeptide. The nonapeptide was treated with trypsin and gave 2 peptides: (V-C-G-A) and (N-FF-W-K). Give the sequence of amino acid in the decapeptide.arrow_forward
- Another strategy used to resolve amino acids involves converting the carboxy group to an ester and then using a chiral carboxylic acid to carry out an acid–base reaction at the free amino group. Using a racemic mixture of alanine enantiomers and (R)-mandelic acid as resolving agent, write out the steps showing how a resolution process would occur.arrow_forwardThe reaction of ninhydrin with an -amino acid occurs in several steps (a) The first step is loss of water to give a triketone. Show the mechanism of the reaction and the structure of the triketone.(b) The second step is formation of an imine by reaction of the amino acid with the triketone. Show its structure. (c) The third step is a decarboxylation. Show the structure of the product and the mechanism of the decarboxylation reaction. (d) The fourth step is hydrolysis of an imine to yield an amine and an aldehyde. Show the structures of both products. (e) The final step is formation of the purple anion. Show the mechanism of the reactionarrow_forwardBrucine is a poisonous alkaloid obtained from Strychnos nux vomica, a tree that grows in India, Sri Lanka, and northern Australia. Write out a resolution scheme, which shows how a racemic mixture of phenylalanine can be resolved using brucine.arrow_forward
- The peptide Proline-Serine-Alanine-Phenylalanine-Glutamine is present at pH 7. Draw the peptide and include stereochemistry.arrow_forwardWhat are the experimental procedures and the laboratory equipments needed to be used in the removing of carbon dioxide and Nucleophilic addition on the nitrogen of isoxazoline that will have a product of protein that contain native serine residues by the reaction of oxazetidine-containing peptides and α-ketoacidarrow_forwardPredict the reactions ofcarbohydrates in acidic and basicsolutions, and with oxidizingand reducing agentsarrow_forward
- Using palmitoleic acid and neglecting stereochemistry,illustrate the reaction of palmitoletic acid with theaddition of brominearrow_forwardProtein: SHAYNERSE Predict the products of the following reactions with the protein given, if there is none, write NO RXN. - Biuret reagent - KOH/Pb(CH2COO)2 - Glyoxilic Acid/Conc. H2SO - Hg/HNO3 - HNO3arrow_forwardSomatostatin is a tetradecapeptide of the hypothalamus that inhibits the release of pituitary growth hormone. Its amino acid sequence has been determined by a combination of Edman degradations and enzymic hydrolysis experiments. On the basis of the following data, deduce the primary structure of somatostatin: 1. Edman degradation gave PTH-Ala. 2. Selective hydrolysis gave peptides having the following indicated sequences: Phe-Trp Thr-Ser-Cys Lys-Thr-Phe Thr-Phe-Thr-Ser-Cys Asn-Phe-Phe-Trp-Lys Ala-Gly-Cys-Lys-Asn-Phe 3. Somatostatin has a disulfide bridge.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





