
(a)
Interpretation:
The mechanism and major product for the given Diels-Alder reaction is to be drawn.
Concept introduction:
The Diels–Alder reaction joins a conjugated diene and a dienophile (either an
Answer to Problem 24.44P
The mechanism and major product for the given Diels-Alder reaction is:
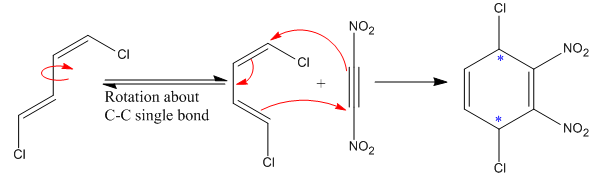
The
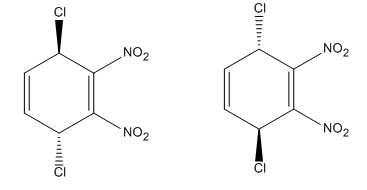
Explanation of Solution
The given reaction is:
Here the diene is in trans configuration. For a Diels–Alder reaction to take place, the diene must be able to attain the

Each end carbon in the diene becomes a chiral center, noted with an asterisk. The

The mechanism and major product for the given Diels-Alder reaction is drawn from the structures of given reactants with stereochemistry.
(b)
Interpretation:
The mechanism and major product for the given Diels-Alder reaction is to be drawn.
Concept introduction:
The Diels–Alder reaction joins a conjugated diene and a dienophile (either an alkene or an alkyne) via the formation of two new
Answer to Problem 24.44P
The mechanism and major product for the given Diels-Alder reaction is:
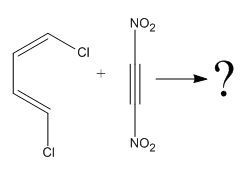
The
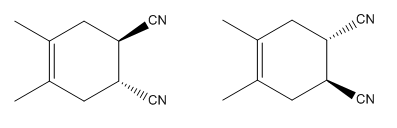
Explanation of Solution
The given reaction is:
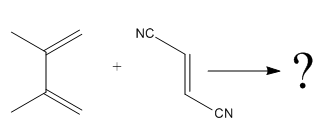
Here the cycloaddition, Diels-Alder reaction forms the six-membered ring product as:

In the above reaction, two carbons in the dienophile become chiral centers, noted with an asterisk.
The

The mechanism and major product for the given Diels-Alder reaction is drawn from the structures of given reactants with stereochemistry.
(c)
Interpretation:
The mechanism and major product for the given Diels-Alder reaction is to be drawn.
Concept introduction:
The Diels–Alder reaction joins a conjugated diene and a dienophile (either an alkene or an alkyne) via the formation of two new
Answer to Problem 24.44P
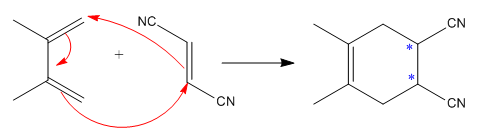
The mechanism and major product for the given Diels-Alder reaction is:
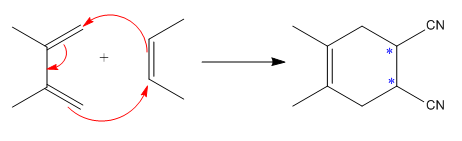
The
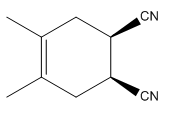
Explanation of Solution
The given reaction is:
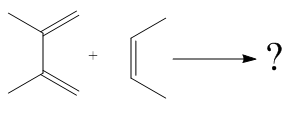
Here the cycloaddition, Diels-Alder reaction forms the six-membered ring product as:

The

The mechanism and major product for the given Diels-Alder reaction is drawn from the structures of given reactants with stereochemistry.
(d)
Interpretation:
The mechanism and major product for the given Diels-Alder reaction is to be drawn.
Concept introduction:
The Diels–Alder reaction joins a conjugated diene and a dienophile (either an alkene or an alkyne) via the formation of two new
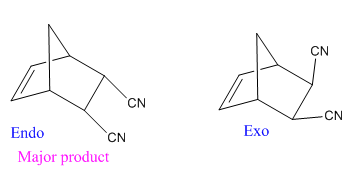
Diels–Alder reactions tend to favor an endo product over an exo product
Answer to Problem 24.44P
The mechanism and major product for the given Diels-Alder reaction is:
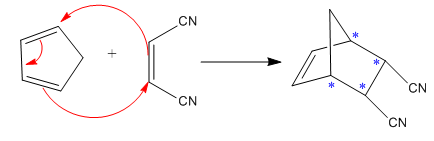
Diels–Alder reactions tend to favor an endo product over an exo product:
Explanation of Solution
The given reaction is:
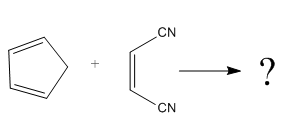
Here the cycloaddition, Diels-Alder reaction forms the bicyclic compound as product:

Here, it is noticed that two carbons of the diene and two carbons of the dienophile become chiral centers, noted with an asterisk.
The

The mechanism and major product for the given Diels-Alder reaction is drawn from the structures of given reactants with stereochemistry.
Want to see more full solutions like this?
Chapter 24 Solutions
ORGANIC CHEMISTRY E-BOOK W/SMARTWORK5
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





