
Organic Chemistry (9th Edition)
9th Edition
ISBN: 9780321971371
Author: Leroy G. Wade, Jan W. Simek
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 24.6, Problem 24.13P
Suggest how you would separate the free i-ammo acid from its acylated D enantiomer in Figure24-5.
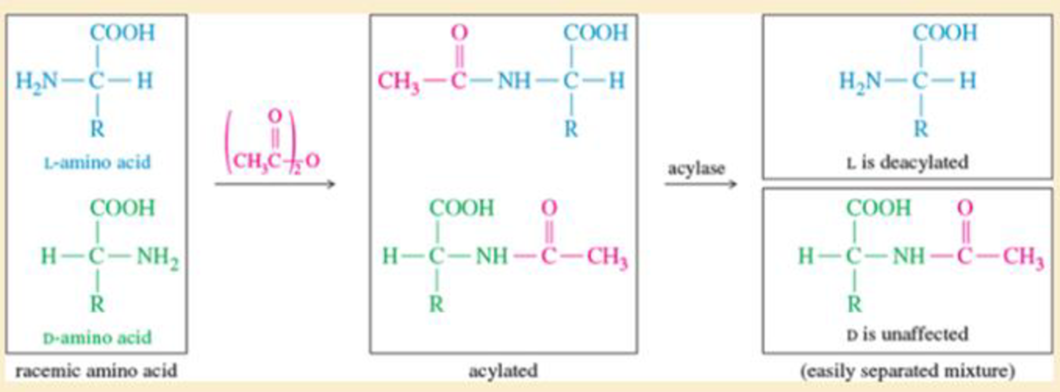
Figure 24-5
Selective enzymatic deacylation. An acylase enzyme (such as hog kidney acylase or carboxypeptidase) deacylates only the natural L-amino acid.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
In 1891, Emil Fischer determined the structures of glucose and the seven other d-aldohexoses using only simple chemical reactions and clever reasoning about stereochemistry andsymmetry. He received the Nobel Prize for this work in 1902. Fischer had determined thatd-glucose is an aldohexose, and he used Ruff degradations to degrade it to (+)-glyceraldehyde. Therefore, the eight d-aldohexose structures shown in Figure 23-3 are the possiblestructures for glucose.Pretend that no names are shown in Figure 23-3 except for glyceraldehyde, and usethe following results to prove which of these structures represent glucose, mannose,arabinose, and erythrose.(a) Upon Ruff degradation, glucose and mannose give the same aldopentose: arabinose.Nitric acid oxidation of arabinose gives an optically active aldaric acid. What are thetwo possible structures of arabinose?(b) Upon Ruff degradation, arabinose gives the aldotetrose erythrose. Nitric acid oxidation of erythrose gives an optically inactive…
In 1891, Emil Fischer determined the structures of glucose and the seven other d-aldohexoses using only simple chemical reactions and clever reasoning about stereochemistry andsymmetry. He received the Nobel Prize for this work in 1902. Fischer had determined thatd-glucose is an aldohexose, and he used Ruff degradations to degrade it to (+)-glyceraldehyde. Therefore, the eight d-aldohexose structures shown in Figure 23-3 are the possiblestructures for glucose.Pretend that no names are shown in Figure 23-3 except for glyceraldehyde, and usethe following results to prove which of these structures represent glucose, mannose,arabinose, and erythrose.(a) Upon Ruff degradation, glucose and mannose give the same aldopentose: arabinose.Nitric acid oxidation of arabinose gives an optically active aldaric acid. What are thetwo possible structures of arabinose?(b) Upon Ruff degradation, arabinose gives the aldotetrose erythrose. Nitric acid oxidation of erythrose gives an optically inactive…
If you had a protein X, which is a soluble enzyme found inside the peroxisome, and you wished to separate it from a similar protein Y, which is an enzyme found embedded in the mitochondrial membrane, what would be your initial techniques for isolating these proteins?
Chapter 24 Solutions
Organic Chemistry (9th Edition)
Ch. 24.2A - Draw three-dimensional representations of the...Ch. 24.2A - Prob. 24.2PCh. 24.2B - The herbicide glyphosate (Roundup) kills plants by...Ch. 24.4 - Draw the structure of the predominant form of a....Ch. 24.4 - Draw the resonance forms of a protonated guanidino...Ch. 24.4 - Although tryptophan contains a heterocyclic amine,...Ch. 24.4 - Prob. 24.7PCh. 24.4 - Prob. 24.8PCh. 24.5A - Show how the following amino acids might be formed...Ch. 24.5B - Prob. 24.10P
Ch. 24.5C - Prob. 24.11PCh. 24.5C - Show how you would use a Strecker synthesis to...Ch. 24.6 - Suggest how you would separate the free i-ammo...Ch. 24.7A - Propose a mechanism for the acid-catalyzed...Ch. 24.7A - Give equations for the formation and...Ch. 24.7B - Prob. 24.16PCh. 24.7C - Prob. 24.17PCh. 24.8B - Draw the complete structures of the following...Ch. 24.9C - Prob. 24.19PCh. 24.9C - Prob. 24.20PCh. 24.9C - Prob. 24.21PCh. 24.9E - Prob. 24.22PCh. 24.9E - Prob. 24.23PCh. 24.10A - Propose a mechanism for the coupling of acetic...Ch. 24.10B - Show how you would synthesize Leu-Gly-Ala-Val-Phe...Ch. 24.10B - Show how solid-phase peptide synthesis would be...Ch. 24 - a. The isoelectric point (pl) of phenylalanine is...Ch. 24 - Prob. 24.28SPCh. 24 - Prob. 24.29SPCh. 24 - Prob. 24.30SPCh. 24 - Prob. 24.31SPCh. 24 - Suggest a method for the synthesis of the...Ch. 24 - Prob. 24.33SPCh. 24 - Write the complete structures for the following...Ch. 24 - The following structure is drawn in an...Ch. 24 - Prob. 24.36SPCh. 24 - Prob. 24.37SPCh. 24 - Show the steps and intermediates in the synthesis...Ch. 24 - Prob. 24.39SPCh. 24 - Lipoic acid is often found near the active sites...Ch. 24 - Prob. 24.41SPCh. 24 - Prob. 24.42SPCh. 24 - Prob. 24.43SPCh. 24 - Complete hydrolysis of an unknown basic...Ch. 24 - Prob. 24.45SPCh. 24 - Prob. 24.46SPCh. 24 - Prob. 24.47SP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Carboxypeptidase requires a Zn²+ cofactor for the hydrolysis of the peptide bond of a C-terminal aromatic amino acid. T/F?arrow_forwardThe overall adenylate kinase reaction (2 ADP = AMP + ATP) is a combination of 3 reactions: ATP hydrolysis, pyrophosphate hydrolysis, and ATP formation. Show each step and provide the standard free energy (ΔG°′) for each and the overall reaction. If the adenylate kinase reaction is at equilibrium and intracellular [ATP] = 5 mM and [ADP] = 0.5 mM, calculate the concentration of AMP at pH 7 and 25 °C. Show all work. ATP (ADP+P) = -30.5 kj/mol; ATP (AMP+PP) = -45.6 kj/mol; PP = -19.2 kj/molarrow_forwardHow many of the -amino acids shown in Table 26-1 contain aromatic rings? How many contain sulfur? How many contain alcohols? How many contain hydrocarbon side chains?arrow_forward
- Draw the structure of the peptide ENDQCW. Draw the stereochemistry of the amino acids. Show the ionization state that would be the major form at pH 1. What is the isoelectric point of the peptide?arrow_forward(a) Figure 23-2 shows that the degradation of d-glucose gives d-arabinose, an aldopentose.Arabinose is most stable in its furanose form. Draw d-arabinofuranose.(b) Ribose, the C2 epimer of arabinose, is most stable in its furanose form. Drawd-ribofuranose.arrow_forwardIs DL-Homocysteine soluble in ether?arrow_forward
- 99. The _____________ plot shows the free energy change for a given amino acid window, allowing the prediction of transmembrane helices.arrow_forward31. ________________ binds to and provides feedback inhibition for ATCase.arrow_forwardChorismate mutase is an enzyme that promotes a pericyclic reaction by forcing the substrate to assume the conformation needed for the reaction. The product of the pericyclic reaction is prephenate that is subsequently converted into the amino acids phenylalanine and tyrosine. What kind of a pericyclic reaction does chorismate mutase catalyze?arrow_forward
- suggest a plausible arrow-pushing mechanism for the formation of a covalent adduct between the suicide inhibitor 5-trifluoromethyluridylic acid and thymidylate synthase that involves the active_site thiolate.arrow_forwardA nonapeptide released by globulins in the blood in response to a waspsting. Hydrolysis gives the following amino acids: 2 Arg, Gly, 2 Phe, 3 Pro, and Ser.Edman degradation gives phenylthiohydantoin of Arg. Cleavage with carboxypeptidase gives Arg. Partial hydrolysis gives the following di- and tripeptides: Phe-Ser, Pro-GlyPhe, Pro-Pro, Ser-Pro-Phe, Phe-Arg, and Arg-Pro. What is the amino acid sequence of this peptide?arrow_forwardWhat are the nonprotein nitrogen components of the blood. Give their chemical structures and relative physiologic concentrations and describe their biosynthesis and excretion. Kindy insert your references. Thank you!arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:9781305446021
Author:Lampman
Publisher:CENGAGE LEARNING - CONSIGNMENT

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
Biomolecules - Protein - Amino acids; Author: Tutorials Point (India) Ltd.;https://www.youtube.com/watch?v=ySNVPDHJ0ek;License: Standard YouTube License, CC-BY