
Organic Chemistry: Principles and Mechanisms (Second Edition)
2nd Edition
ISBN: 9780393663556
Author: Joel Karty
Publisher: W. W. Norton & Company
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 25, Problem 25.2YT
Interpretation Introduction
Interpretation:
By comparing the bond energies of the given bonds to that of C-H bond, it is to be verified that halogens and peroxides have weak bonds.
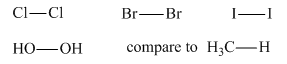
Concept introduction:
The energy that would be required to increase the distance between two bonded atoms from the bond length to infinity, that is, breaking of one mole of molecules into their individual atoms, is termed as the bond energy. The larger value of bond energy represents strong bond.
Table: Homolytic Bond Dissociation Energies
| Bond | Homolytic |
| 243 | |
| 192 | |
| 151 | |
| 211 | |
| 439 |
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
refer to image please please label graph and show all work. CALCULATE R VALUE AND EXPLAIN.
will only rate if graph is labelled.
label name of structure too.
Draw two other possible resonance structures and the mechanism arrows to get from one to the next. You will have to draw any lone pairs that you use. Also you have to go from one contributor
to the next in a linear fashion meaning that one structure should not be used to get to two different
contributors. Lastly circle the major contributor(s). There are actually five contributors but you just have to draw two.
I already know this is a schmdit reaction, I need to know what extact bonds are broken formed please :)))
Chapter 25 Solutions
Organic Chemistry: Principles and Mechanisms (Second Edition)
Ch. 25 - Prob. 25.1PCh. 25 - Prob. 25.2PCh. 25 - Prob. 25.3PCh. 25 - Prob. 25.4PCh. 25 - Prob. 25.5PCh. 25 - Prob. 25.6PCh. 25 - Prob. 25.7PCh. 25 - Prob. 25.8PCh. 25 - Prob. 25.9PCh. 25 - Prob. 25.10P
Ch. 25 - Prob. 25.11PCh. 25 - Prob. 25.12PCh. 25 - Prob. 25.13PCh. 25 - Prob. 25.14PCh. 25 - Prob. 25.15PCh. 25 - Prob. 25.16PCh. 25 - Prob. 25.17PCh. 25 - Prob. 25.18PCh. 25 - Prob. 25.19PCh. 25 - Prob. 25.20PCh. 25 - Prob. 25.21PCh. 25 - Prob. 25.22PCh. 25 - Prob. 25.23PCh. 25 - Prob. 25.24PCh. 25 - Prob. 25.25PCh. 25 - Prob. 25.26PCh. 25 - Prob. 25.27PCh. 25 - Prob. 25.28PCh. 25 - Prob. 25.29PCh. 25 - Prob. 25.30PCh. 25 - Prob. 25.31PCh. 25 - Prob. 25.32PCh. 25 - Prob. 25.33PCh. 25 - Prob. 25.34PCh. 25 - Prob. 25.35PCh. 25 - Prob. 25.36PCh. 25 - Prob. 25.37PCh. 25 - Prob. 25.38PCh. 25 - Prob. 25.39PCh. 25 - Prob. 25.40PCh. 25 - Prob. 25.41PCh. 25 - Prob. 25.42PCh. 25 - Prob. 25.43PCh. 25 - Prob. 25.44PCh. 25 - Prob. 25.45PCh. 25 - Prob. 25.46PCh. 25 - Prob. 25.47PCh. 25 - Prob. 25.48PCh. 25 - Prob. 25.49PCh. 25 - Prob. 25.50PCh. 25 - Prob. 25.51PCh. 25 - Prob. 25.52PCh. 25 - Prob. 25.53PCh. 25 - Prob. 25.54PCh. 25 - Prob. 25.55PCh. 25 - Prob. 25.56PCh. 25 - Prob. 25.57PCh. 25 - Prob. 25.58PCh. 25 - Prob. 25.59PCh. 25 - Prob. 25.60PCh. 25 - Prob. 25.61PCh. 25 - Prob. 25.62PCh. 25 - Prob. 25.63PCh. 25 - Prob. 25.64PCh. 25 - Prob. 25.65PCh. 25 - Prob. 25.66PCh. 25 - Prob. 25.67PCh. 25 - Prob. 25.68PCh. 25 - Prob. 25.69PCh. 25 - Prob. 25.70PCh. 25 - Prob. 25.71PCh. 25 - Prob. 25.72PCh. 25 - Prob. 25.73PCh. 25 - Prob. 25.74PCh. 25 - Prob. 25.75PCh. 25 - Prob. 25.76PCh. 25 - Prob. 25.77PCh. 25 - Prob. 25.1YTCh. 25 - Prob. 25.2YTCh. 25 - Prob. 25.3YTCh. 25 - Prob. 25.4YTCh. 25 - Prob. 25.5YTCh. 25 - Prob. 25.6YTCh. 25 - Prob. 25.7YTCh. 25 - Prob. 25.8YTCh. 25 - Prob. 25.9YTCh. 25 - Prob. 25.10YTCh. 25 - Prob. 25.11YTCh. 25 - Prob. 25.12YTCh. 25 - Prob. 25.13YTCh. 25 - Prob. 25.14YTCh. 25 - Prob. 25.15YTCh. 25 - Prob. 25.16YTCh. 25 - Prob. 25.17YTCh. 25 - Prob. 25.18YTCh. 25 - Prob. 25.19YTCh. 25 - Prob. 25.20YTCh. 25 - Prob. 25.21YTCh. 25 - Prob. 25.22YTCh. 25 - Prob. 25.23YTCh. 25 - Prob. 25.24YT
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- I need help creating a detailed electron pushing mechanism fkr this reaction. It needs to be done as an SN2 reaction. Please help.arrow_forwardcan you show all the mechanism arrows please? Im not sure how to correctly take the OMgBr off , and how the OH turned into a double bondarrow_forwardDraw the resonance forms for the intermediate generated by an electrophile adding to anisole. Consider all of the positions where the E+ could add. Which is preferred ortho, meta or para addition? Is my work correct?arrow_forward
- 1 example for organic rearrangement reaction show: a. the overall reaction (reactants --> products) b. the reaction mechanism (indicate intermediate product) c. indicate which is the reactive species or intermediate in the reaction (radical? electrophile? nucleophile?) d. overall description of the reaction eg., radical substitution or SRarrow_forwardWhich rxn would be fastest? Which would be slowest?arrow_forwardPlease help me with the organic chemistry problem below: Consider the reaction below: (Check the attached image) (it is between Furan and maleic anhydride, a DIels-Alder reaction) a) Will this reaction for an endo product (with a melting point of 80-81 degrees) or the exo product (with a melitng point of 114 degrees)? b) Carefully explain why the product must have been formed the way it did (exo or endo). c) Provide a mechanism for this reaction.arrow_forward
- Plz do Asap...! With proper explanation and reaction ...!arrow_forwardPlease help me making a sheet look like the 1st picture for ethane using alkenes, alkenes, alkynes but it should only have the reaction shown in second picture please make it loke a sheet ASAParrow_forwardAnswer is Reaction 7? Can you explain to me, please?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning