
Concept explainers
(a)
To determine: The structure and name of any eight isomers among the eighteen isomers of
Interpretation: The structure and name of any eight isomers among the eighteen isomers of alkane
Concept introduction: The rules for the naming of cycloalkanes are stated below.
- If two different cycloalkanes are present then the one with the more number of carbon atoms act as the parent chain.
- If the side chain has more number of carbon atoms then it act as the parent chain and the cyclic group becomes the substituent and is ended with the word”-yl”.
- The numbering is done in such a manner that the substituents groups occupy the lowest position.
- If different types of substituent groups are present then they are written in an alphabetical order.
- If a substituent is present more than one time then prefixes like di, tri, tetra are used depending on the number of times that particular substituent group appears in the given compound.
- If geometrical isomerism is possible in the given compound then cis and trans is used before the name of the compound.
(a)
Answer to Problem 3.33SP
The structure and name of any eight isomers among the eighteen isomers of alkane
Explanation of Solution
The isomers of given alkane
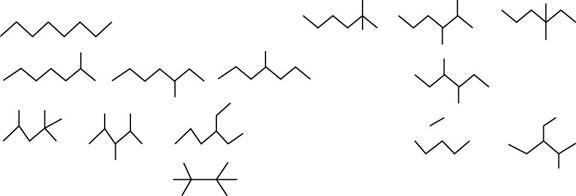
Figure 1
The randomly selected first isomer is shown below.
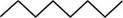
Figure 2
The name of the above compound is octane.
The randomly selected second isomer is shown below.
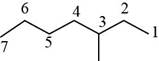
Figure 3
The name of the above compound is
The randomly selected third isomer is shown below.
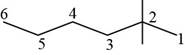
Figure 4
The name of the above compound is
The randomly selected fourth isomer is shown below.
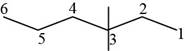
Figure 5
The name of the above compound is
The randomly selected fifth isomer is shown below.
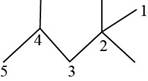
Figure 6
The name of the above compound is
The randomly selected sixth isomer is shown below.
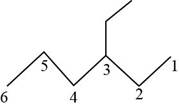
Figure 7
The name of the above compound is
The randomly selected seventh isomer is shown below.
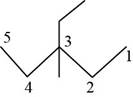
Figure 8
The name of the above compound is
The randomly selected seventh isomer is shown below.
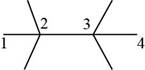
Figure 9
The name of the above compound is
(b)
To determine: The structure and name of six isomeric cyclopentanes having molecular formula
Interpretation: The structure and name of six isomeric cyclopentanes having molecular formula
Concept introduction: The rules for the naming of cycloalkanes are stated below.
- If two different cycloalkanes are present then the one with the more number of carbon atoms act as the parent chain.
- If the side chain has more number of carbon atoms then it act as the parent chain and the cyclic group becomes the substituent and is ended with the word”-yl”.
- The numbering is done in such a manner that the substituents groups occupy the lowest position.
- If different types of substituent groups are present then they are written in an alphabetical order.
- If a substituent is present more than one time then prefixes like di, tri, tetra are used depending on the number of times that particular substituent group appears in the given compound.
- If geometrical isomerism is possible in the given compound then cis and trans is used before the name of the compound.
(b)
Answer to Problem 3.33SP
The structure and name of six isomeric cyclopentanes having molecular formula
Explanation of Solution
The isomers of given cyclopentane
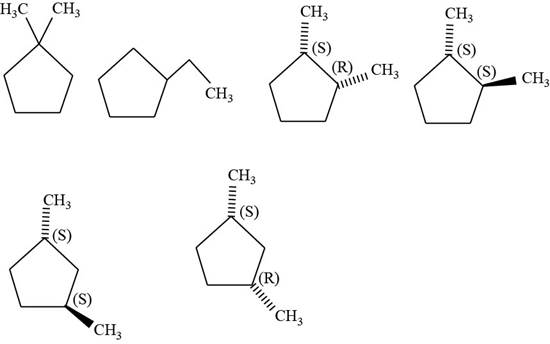
Figure 10
The first isomer is shown below.
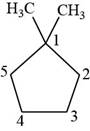
Figure 11
According to IUPAC rules numbering is started from the substituted carbon. Therefore, the name of the above compound is
The second isomer is shown below.
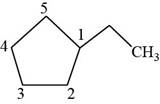
Figure 12
According to IUPAC rules numbering is started from the substituted carbon. Therefore, the name of the above compound is
The third isomer is shown below.
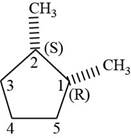
Figure 13
According to IUPAC rules numbering is started from the substituted carbon. Numbering is done in such a manner that the substituents occupy the least position. On the basis of priority of groups
The fourth isomer is shown below.
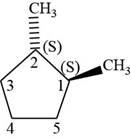
Figure 14
According to IUPAC rules numbering is started from the substituted carbon. Numbering is done in such a manner that the substituents occupy the least position. On the basis of priority of groups
The fifth isomer is shown below.
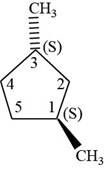
Figure 15
According to IUPAC rules numbering is started from the substituted carbon. Numbering is done in such a manner that the substituents occupy the least position. On the basis of priority of groups
The sixth isomer is shown below.
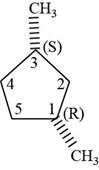
Figure 16
According to IUPAC rules numbering is started from the substituted carbon. Numbering is done in such a manner that the substituents occupy the least position. On the basis of priority of groups
Want to see more full solutions like this?
Chapter 3 Solutions
Organic Chemistry (9th Edition)
- Following is a structural formula for cortisol (hydrocortisone). Draw a stereo-representation of this molecule showing the conformations of the five- and six-membered rings.arrow_forwardRead Appendix B on naming branched alkyl substituents, and draw all possible alkyl groups having the formula C5H11–. Give the IUPAC names for the eight compounds of molecular formula C10H20 that contain a cyclopentane ring with each of these alkyl groups as a substituent.arrow_forwardOrganic Chemistry HW: CANNOT BE HAND DRAWN 2,6-dimethyloct-2-ene Provide a detailed typed explanation of Stereoisomers show the expanded structure of your molecule. Calculate the maximum number of possible stereoisomers of your molecule using the following formula: Maximum number of possible stereoisomers = 2n (where n= the number of chiral carbons in your molecule). This calculation does not include E- or Z- isomers for any compounds containing double bonds Type or using a computer program "draw" the possible stereoisomers of the molecule. Note that E-, Z- isomers of each stereoisomer are also possible and would not be accounted for by the formula above; draw any E- or Z- isomers.arrow_forward
- Explain Conformations of Acyclic Alkanes—Ethane ?arrow_forwardBased on naming branched alkyl substituents, and draw all possible alkyl groups having the formula C5H11–. Give the IUPAC names for the eight compounds of molecular formula C10H20that contain a cyclopentane ring with each of these alkyl groups as a substituent.arrow_forwardDraw the line bond structures for the following alkenes, cyclic alkenes, and alkynes: Can you explain to me about this part A) noncyclic alkenes that contain 4 carbon atoms (3 possible), please? Can you explain to me about this part B) cyclic alkenes that contain 4 carbon atoms (4 possible), please? Can you explain to me about this part C) alkynes that contain 4 carbon atoms (2 possible, neither of them is a cyclic alkyne), please?arrow_forward
- Draw three constitutional isomers having molecular formula C7H14 that contains a five-membered ring and two methyl groups bonded to that ring.arrow_forwardWhy is a substituted cyclohexane ring more stable with a larger group in the equatorial position?arrow_forwardDraw and name a constitutional isomer of C9H18 that has cis/trans isomers and is named a “3-hexene”. (Label your structure with the proper E/Z nomenclature).arrow_forward
- Draw all the isomers with molecular formula C6H12 that contain a cyclobutane ring. (Hint: There are seven.)arrow_forwardThere are 18 isomeric alkanes of molecular formula C8H18. Draw and name any eight of them.arrow_forwardWhich alkenes show cis,trans isomerism? For each alkene that does, draw the trans isomer. Q.) 2-Pentenearrow_forward
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning



