
Concept explainers
For each of the following molecules or ions that contain sulfur, write the Lewis structure(s), predict the molecular structure (including bond angles), and give the expected hybrid orbitals for sulfur.
a. SO2
b. SO3
c. 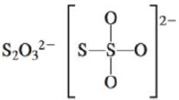
d. 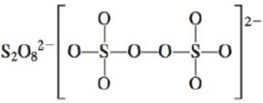
e. SO32−
f. SO42−
g. SF2
h. SF4
i. SF6
j. F3S—SF
k. SF5+
(a)
Interpretation: The Lewis dot structure, molecular geometry, bond angles of the given molecules and expected hybrid orbitals for sulfur is to be stated.
Concept introduction: When the atomic orbitals overlap with each other in the region where density of electrons is high, then molecular orbitals are formed. Overlap of the atomic orbitals determines the efficiency of the interaction between the atomic orbitals.
Energy of bonding molecular orbitals is less than the nonbonding molecular orbitals.
To determine: The Lewis dot structure, molecular geometry, bond angles and expected hybrid orbitals for sulfur in
Explanation of Solution
Explanation
There are six valence electrons on each sulfur and oxygen atom. Two oxygen atoms are bonded to sulfur atom. Therefore, the total valence electrons are
Therefore the geometry is bent. The bond angle is less than
The Lewis structure of
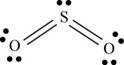
Figure 1
(b)
Interpretation: The Lewis dot structure, molecular geometry, bond angles of the given molecules and expected hybrid orbitals for sulfur is to be stated.
Concept introduction: When the atomic orbitals overlap with each other in the region where density of electrons is high, then molecular orbitals are formed. Overlap of the atomic orbitals determines the efficiency of the interaction between the atomic orbitals.
Energy of bonding molecular orbitals is less than the nonbonding molecular orbitals.
To determine: The Lewis dot structure, molecular geometry, bond angles and expected hybrid orbitals for sulfur in
Explanation of Solution
Explanation
There are six valence electrons on sulfur and each oxygen atom. Two oxygen atoms are attached to sulfur, therefore, the total number of valence electrons is
The molecule has trigonal planar geometry with bond angle
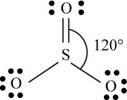
Figure 2
(c)
Interpretation: The Lewis dot structure, molecular geometry, bond angles of the given molecules and expected hybrid orbitals for sulfur is to be stated.
Concept introduction: When the atomic orbitals overlap with each other in the region where density of electrons is high, then molecular orbitals are formed. Overlap of the atomic orbitals determines the efficiency of the interaction between the atomic orbitals.
Energy of bonding molecular orbitals is less than the nonbonding molecular orbitals.
To determine: The Lewis dot structure, molecular geometry, bond angles and expected hybrid orbitals for sulfur in
Explanation of Solution
Explanation
There are six valence electrons on sulfur and each oxygen atom. Three oxygen atoms and one sulfur atom is attached to central sulfur atom and charge on the molecule is
By bonding in this way, they complete their octet. The molecular structure is tetrahedral with bond angle approximately equal to
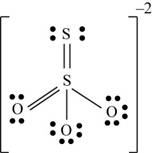
Figure 3
(d)
Interpretation: The Lewis dot structure, molecular geometry, bond angles of the given molecules and expected hybrid orbitals for sulfur is to be stated.
Concept introduction: When the atomic orbitals overlap with each other in the region where density of electrons is high, then molecular orbitals are formed. Overlap of the atomic orbitals determines the efficiency of the interaction between the atomic orbitals.
Energy of bonding molecular orbitals is less than the nonbonding molecular orbitals.
To determine: The Lewis dot structure, molecular geometry, bond angles and expected hybrid orbitals for sulfur in
Explanation of Solution
Explanation
There are six valence electrons on sulfur and each oxygen atom. There are eight oxygen atoms and two sulfur atoms are present in the molecule and charge on the molecule is
The two oxygen atoms in the centre are bonded by single bond. By bonding in this way, they complete their octet. The molecular structure is tetrahedral with bond angle approximately equal to
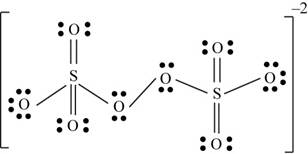
Figure 4
(e)
Interpretation: The Lewis dot structure, molecular geometry, bond angles of the given molecules and expected hybrid orbitals for sulfur is to be stated.
Concept introduction: When the atomic orbitals overlap with each other in the region where density of electrons is high, then molecular orbitals are formed. Overlap of the atomic orbitals determines the efficiency of the interaction between the atomic orbitals.
Energy of bonding molecular orbitals is less than the nonbonding molecular orbitals.
To determine: The Lewis dot structure, molecular geometry, bond angles and expected hybrid orbitals for sulfur in
Explanation of Solution
Explanation
There are six valence electrons on sulfur and each oxygen atom. Three oxygen atoms and one sulfur atom present in the molecule and charge on the molecule is
One oxygen atom is single bonded with sulfur and one is joined by pi bond. By bonding in this way, they complete their octet. The molecular structure is trigonal pyramidal with bond angle approximately equal to
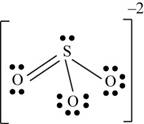
Figure 5
(f)
Interpretation: The Lewis dot structure, molecular geometry, bond angles of the given molecules and expected hybrid orbitals for sulfur is to be stated.
Concept introduction: When the atomic orbitals overlap with each other in the region where density of electrons is high, then molecular orbitals are formed. Overlap of the atomic orbitals determines the efficiency of the interaction between the atomic orbitals.
Energy of bonding molecular orbitals is less than the nonbonding molecular orbitals.
To determine: The Lewis dot structure, molecular geometry, bond angles and expected hybrid orbitals for sulfur in
Explanation of Solution
Explanation
There are six valence electrons on sulfur and each oxygen atom. Four oxygen atoms and one sulfur atom is present in the molecule and charge on the molecule is
Two oxygen atoms are single bonded with sulfur and two joined by pi bond. By bonding in this way, they complete their octet. The molecular structure is tetrahedral with bond angle approximately equal to
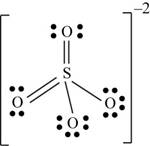
Figure 6
(g)
Interpretation: The Lewis dot structure, molecular geometry, bond angles of the given molecules and expected hybrid orbitals for sulfur is to be stated.
Concept introduction: When the atomic orbitals overlap with each other in the region where density of electrons is high, then molecular orbitals are formed. Overlap of the atomic orbitals determines the efficiency of the interaction between the atomic orbitals.
Energy of bonding molecular orbitals is less than the nonbonding molecular orbitals.
To determine: The Lewis dot structure, molecular geometry, bond angles and expected hybrid orbitals for sulfur in
Explanation of Solution
Explanation
There are six valence electrons on sulfur and seven valence electrons on each fluorine atom. Two fluorine atoms and one sulfur atom is present in the molecule, therefore, the total number of valence electrons is
The sulfur is bonded to two fluorine atoms by sigma bond. By bonding in this way, they complete their octet. The molecular structure is bent due to presence of lone pairs of electrons on sulfur. The bond angle is less than
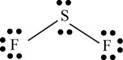
Figure 7
(h)
Interpretation: The Lewis dot structure, molecular geometry, bond angles of the given molecules and expected hybrid orbitals for sulfur is to be stated.
Concept introduction: When the atomic orbitals overlap with each other in the region where density of electrons is high, then molecular orbitals are formed. Overlap of the atomic orbitals determines the efficiency of the interaction between the atomic orbitals.
Energy of bonding molecular orbitals is less than the nonbonding molecular orbitals.
To determine: The Lewis dot structure, molecular geometry, bond angles and expected hybrid orbitals for sulfur in
Explanation of Solution
Explanation
There are six valence electrons on sulfur and seven valence electrons on each fluorine atom. Four fluorine atoms and one sulfur atom is present in the molecule, therefore, the total number of valence electrons is
The molecular structure is see-saw due to presence of lone pair of electrons on sulfur. The equatorial bond angles are
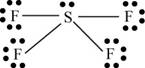
Figure 8
(i)
Interpretation: The Lewis dot structure, molecular geometry, bond angles of the given molecules and expected hybrid orbitals for sulfur is to be stated.
Concept introduction: When the atomic orbitals overlap with each other in the region where density of electrons is high, then molecular orbitals are formed. Overlap of the atomic orbitals determines the efficiency of the interaction between the atomic orbitals.
Energy of bonding molecular orbitals is less than the nonbonding molecular orbitals.
To determine: The Lewis dot structure, molecular geometry, bond angles and expected hybrid orbitals for sulfur in
Explanation of Solution
Explanation
There are six valence electrons on sulfur and seven valence electrons on each fluorine atom. Six fluorine atoms and one sulfur atom is present in the molecule, therefore, the total number of valence electrons is
The molecular structure is octahedral with bond angle
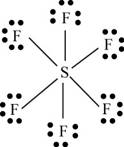
Figure 9
(j)
Interpretation: The Lewis dot structure, molecular geometry, bond angles of the given molecules and expected hybrid orbitals for sulfur is to be stated.
Concept introduction: When the atomic orbitals overlap with each other in the region where density of electrons is high, then molecular orbitals are formed. Overlap of the atomic orbitals determines the efficiency of the interaction between the atomic orbitals.
Energy of bonding molecular orbitals is less than the nonbonding molecular orbitals.
To determine: The Lewis dot structure, molecular geometry, bond angles and expected hybrid orbitals for sulfur in
Explanation of Solution
Explanation
There are six valence electrons on sulfur and seven valence electrons on each fluorine atom. Four fluorine atoms and two sulfur atoms are present in the molecule, therefore, the total number of valence electrons is
The molecular structure is see-saw due to presence of lone pair of electrons on sulfur. The equatorial bond angles are
The Lewis structure of
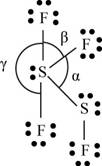
Figure 10
(k)
Interpretation: The Lewis dot structure, molecular geometry, bond angles of the given molecules and expected hybrid orbitals for sulfur is to be stated.
Concept introduction: When the atomic orbitals overlap with each other in the region where density of electrons is high, then molecular orbitals are formed. Overlap of the atomic orbitals determines the efficiency of the interaction between the atomic orbitals.
Energy of bonding molecular orbitals is less than the nonbonding molecular orbitals.
To determine: The Lewis dot structure, molecular geometry, bond angles and expected hybrid orbitals for sulfur in
Explanation of Solution
Explanation
There are six valence electrons on sulfur and seven valence electrons on each fluorine atom. Five fluorine atoms and one sulfur atom is present in the molecule and charge on the molecule is
The molecular structure is trigonal bipyramidal with equatorial bond angles
The Lewis structure of
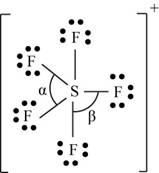
Figure 11
Want to see more full solutions like this?
Chapter 4 Solutions
Chemistry: An Atoms First Approach
- The antibiotic thiarubin-A was discovered by studying the feeding habits of wild chimpanzees in Tanzania. The structure for thiarubin-A is a. Complete the Lewis structure, showing all lone pairs of electrons. b. Indicate the hybrid orbitals used by the carbon and sulfur atoms in thiarubin-A. c. How many and bonds are present in this molecule?arrow_forwardGive the expected hybridization of the central atom for the molecules or ions in Exercises 81 and 87 from Chapter 3.arrow_forwardPredict the valence electron molecular orbital configurations for the following, and state whether they will be stable or unstable ions. (a) Na,2+ (b) Mg,2 (c) AI,2 (d) Si,2 (e) p2+ (f) s,2 (g) F,2 (h) Ar,2 40. Predict the valence electron molecular orbital configurations for the following, and state whether they will be stable or unstable ions. (a) Na22+ (b) Mg22+ (c) Al22+ (d) Si22+ (e) P22+ (f) S22+ (g) F22+ (h) Ar22+arrow_forward
- What is the electron pair geometry, lewis structure, molecular geometry, and hybridization of HF? Is it polar or non-polar?arrow_forwardBriefly explain how SF6 can be formed while the octet rule only allows for eight electrons. Use the conventional explanation and compare it with the molecular orbital theoryarrow_forward4.Examine the model and real structures for SO2. A.In what way does the central atom violate the octet rule? When is it possible for molecules to have this exception to the octet rule? B.Calculate the difference in the bond angle between the real and model structure? Is this difference larger or smaller than the difference observed for H2O? Why is the deviation between the real and model structure different for SO2compared to H2O? The deviation for H2O is 5 degreesarrow_forward
- What are the electron-pair geometry and the molecular structure of each of the following molecules or ions?(a) ClF5(b) ClO2−(c) TeCl42−(d) PCl3(e) SeF4(f) PH2−arrow_forwardConsider the SCl2 molecule. (a) What is the electron configuration of an isolated S atom? (b) What is the electron configuration of an isolated Cl atom? (c) What hybrid orbitals should be constructed on the S atom to make the S-Cl bonds in SCl2 ? (d) What valence orbitals if any, remain unhybridized on the S atom in SCL2 ?arrow_forwardWhich hybridization scheme in Valence Bond theory gives rise to: trigonal planar electronic geometry? octahedral electronic geometry? tetrahedral electronic geometry? trigonal bipyramidal electronic geometry? linear electronic geometry? Use notation: sp, sp2, sp3, dsp3, or d2sp3.arrow_forward
- The molecular orbital valence electron configuration of the species B2+ is shown. Determine the total number of valence electrons in the species B2+.arrow_forwardWhat are the hybridizations of each one? :)arrow_forwardA useful solvent that will dissolve salts as well as organic compounds is the compound acetonitrile, H3CCN. It is present in paint strippers.(a) Write the Lewis structure for acetonitrile, and indicate the direction of the dipole moment in the molecule.(b) Identify the hybrid orbitals used by the carbon atoms in the molecule to form σ bonds.(c) Describe the atomic orbitals that form the π bonds in the molecule. Note that it is not necessary to hybridize the nitrogen atom.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning





