
(a)
Interpretation: The solubility of
Concept Introduction:A solution is a mixture of solute and solvent. The solubility of solute in solvent depends on the interaction between solute and solvent. It also depends on the polarity of molecules also. As the polar and ionic compounds are soluble in polar solvents whereas the non-polar compounds are soluble in non-polar solvents such as organic solvents. Therefore, it is said to be “Like dissolves Like”.
(a)
Answer to Problem 6E
Explanation of Solution
(b)
Interpretation: The solubility of naphthalene in organic solvent or water needs to be explained.
Concept Introduction:A solution is a mixture of solute and solvent. The solubility of solute in solvent depends on the interaction between solute and solvent. It also depends on the polarity of molecules also. As the polar and ionic compounds are soluble in polar solvents whereas the non-polar compounds are soluble in non-polar solvents such as organic solvents. Therefore, it is said to be “Like dissolves Like”.
(b)
Answer to Problem 6E
Naphthalene is soluble in organic solvent as it is a non-polar compound.
Explanation of Solution
Naphthalene is an aromatic hydrocarbon which is composed of C and H atoms. The structural formula of naphthalene can be shown as below:
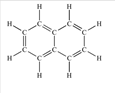
Since there is no other functional group in the molecule,therefore, it is a non-polar molecule and is soluble in a non-polar solvent.
(c)
Interpretation: The solubility of Anthracenein organic solvent or water needs to be explained.
Concept Introduction:A solution is a mixture of solute and solvent. The solubility of solute in solvent depends on the interaction between solute and solvent. It also depends on the polarity of molecules also. As the polar and ionic compounds are soluble in polar solvents whereas the non-polar compounds are soluble in non-polar solvents such as organic solvents. Therefore, it is said to be “Like dissolves Like”.
(c)
Answer to Problem 6E
Anthracene is soluble in organic solvent as it is a non-polar compound.
Explanation of Solution
Anthracene is an aromatic hydrocarbon which is composed of C and H atoms. The structural formula of Anthracene can be shown as below:
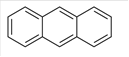
Since there is no other functional group in the molecule therefore it is a non-polar molecule and is soluble in a non-polar solvent.
(d)
Interpretation: The solubility of phenolin organic solvent or water needs to be explained.
Concept Introduction:A solution is a mixture of solute and solvent. The solubility of solute in solvent depends on the interaction between solute and solvent. It also depends on the polarity of molecules also. The polar and ionic compounds are soluble in polar solvents, whereas the non-polar compounds are soluble in non-polar solvents such as organic solvents. Therefore, it is said to be “Like dissolves Like”.
(d)
Answer to Problem 6E
Phenol is soluble water as it is a polar compound.
Explanation of Solution
Phenol is an aromatic hydrocarbon which is composed of C and H atoms with −OH group. The structural formula of phenol can be shown as below:
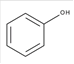
The −OH group can form hydrogen bond with water molecule therefore it is soluble in water.
(e)
Interpretation: The solubility of
Concept Introduction:A solution is a mixture of solute and solvent. The solubility of solute in solvent depends on the interaction between solute and solvent. It also depends on the polarity of molecules also. The polar and ionic compounds are soluble in polar solvents, whereas, the non-polar compounds are soluble in non-polar solvents such as organic solvents. Therefore, it is said to be “Like dissolves Like”.
(e)
Answer to Problem 6E
Explanation of Solution
can be shown as below:
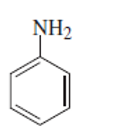
(f)
Interpretation: The solubility of
Concept Introduction:A solution is a mixture of solute and solvent. The solubility of solute in solvent depends on the interaction between solute and solvent. It also depends on the polarity of molecules also. The polar and ionic compounds are soluble in polar solvents, whereas, the non-polar compounds are soluble in non-polar solvents such as organic solvents. Therefore, it is said to be “Like dissolves Like”.
(f)
Answer to Problem 6E
Explanation of Solution
(h)
Interpretation: The solubility of
Concept Introduction:A solution is a mixture of solute and solvent. The solubility of solute in solvent depends on the interaction between solute and solvent. It also depends on the polarity of molecules also. The polar and ionic compounds are soluble in polar solvents, whereas, the non-polar compounds are soluble in non-polar solvents such as organic solvents. Therefore, it is said to be “Like dissolves Like”.
(h)
Answer to Problem 6E
Explanation of Solution
Want to see more full solutions like this?
Chapter 5 Solutions
EBK EXPERIMENTAL ORGANIC CHEMISTRY: A M
- the eugenol and water distillate you collect is apt to be cloudy, particularly the first part of this distillate. Explain why.arrow_forwardA mixture of p-nitrophenol and o-nitrophenol can be separated by steam distillation. The o-nitrophenol is steam volatile, and the para isomer is not volatile. Explain. Base your answer on the ability of the isomers to form hydrogen bonds internally.arrow_forward1. The reactant (2-methyl-2-butanol) has a limited solubility in water at room temperature. 10.0 mL of 2-methyl-2-butanol will dissolve in ~ 80 mL of water. In this experiment, 10.0 mL of 2-methyl-2-butanol readily dissolved in 20 mL of 12 mol/L aqueous HCl. a. Why does this compound exhibit some solubility in water? Consider intermolecular forces and include a diagram to illustrate the relevant intermolecular forces. Why is it not completely miscible in water (in all proportions)?arrow_forward
- In extraction process, the solution contains mixture of benzoic acid and sodium chloride and was heated with tap water, chloroform was added in the separatory funnel. Why is it not possible to use alcohol instead of chloroform in this procedure?arrow_forwardAssuming that either solvent is otherwise acceptable in a given instance, what advantages does ethanol have over 1-octanol as a crystallization solvent? Hexane over pentane? Water over methanol?arrow_forwardWhat trend is there as you increase the water content of the solvent system in an SN1reaction? Explain.arrow_forward
- Assume that charcoal and sugar are the two main impurities in the sample of benzoic acid. Explain how you could perform recrystallization using water toremove each impurity.arrow_forwardWhy is benzoic acid more soluble in toulene han hexanearrow_forwardThere should be a difference in your results between the solubilities of benzophenone in methyl alcohol and benzophenone in hexane. Explain this difference.arrow_forward
- Describe how an emulsion could break down in case it forms in an acid-base extraction or in general.arrow_forwardAn unknown sample is found to be soluble in water. The aqueous solution was found to change blue litmus paper to red and it gave a red precipitate with Benedict’s reagent. How many functional groups are present in this sample and what are their identities? There are two explanations as to why these functional groups are present in this sample. What are these two explanations?arrow_forwardIf we are recrystallizing a PURE compound, what will happen to the sample if we cool the solution rapidly, i.e., placing it on an ice bath without cooling it first at room temperature? Note: kindly disregard the effect of rapid cooling on the container used.arrow_forward
 EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole

