
(a)
Interpretation: The acid base reaction of aniline with hydrochloric acid needs to be explained.
Concept Introduction:Bronsted and Lowery purposed the Bronsted-Lowry acid-base theory. It states that acid can give
(b)
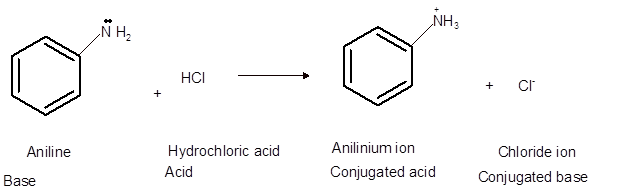
Interpretation: The acid, base, conjugated acid and conjugated base in the reaction of aniline with hydrochloric acid needs to be determined.
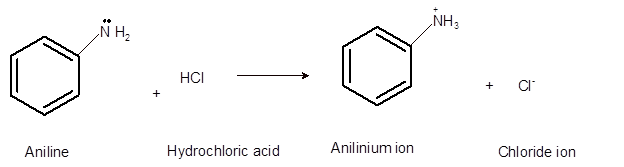
Concept Introduction:Bronsted and Lowery purposed the Bronsted-Lowry acid-base theory. It states that acid can give
(c)
Interpretation: The solubility of aniline and its conjugated base in diethyl ether and water needs to be explained.
Concept Introduction:Bronsted and Lowery purposed the Bronsted-Lowry acid-base theory. It states that acid can give
Want to see the full answer?
Check out a sample textbook solution
Chapter 5 Solutions
EBK EXPERIMENTAL ORGANIC CHEMISTRY: A M
- A mixture of NaCl, SiO2, and CaCO3 is separated following the procedure given in this experiment. Indicate how each of the following procedural changes would affect the amount of the specified substance recovered. Briefly explain. Hint: Refer to Table 1 for Solubility & Reactivity The recovery of CaCO3 was attempted by adding 3M H2SO4 instead of 3M HCl to the SiO2/CaCO3 residue.arrow_forwardHow do the reactions of phenol samples with FeCl3compare? Which structural component of the phenols account for the observation? 2. What compound is the precipitate formed in the Bromine water test? 3. Write the reaction formed in the formation of phenolphthalein. Identify the functional group in phenolphthalein, which is responsible for the indicator property. 4. What is the significance of Millon's test?arrow_forward10.0 mL of 0.100 M HCl solution was added to 20.0 mL of 0.100 M (CH3CH2)3N (triethylamine). Kb (CH3CH2)3N = 5.2 x 10–4 Determine the following: a) Concentration of (CH3CH2)3N that did not react with acid. b) Concentration of (CH3CH2)3NH+ formed from the reaction with acid. c) pOH.arrow_forward
- Describe why the inside wall of the flask is rinsed with deionized water during accurate titration. Does this washing affect the molar quantity of acetic acid in the sample? Describe..?arrow_forwardFor acid-base extractions: Explain what result would you expect if you accidentally extracted the mixture in the reverse order (HCl, NaOH, NaHCO3)? [instead of using NaHCO3 first, NaOH second, and then HCl third]arrow_forwardSuppose you made a mistake in Ag group analysis , and instead of 6M HCl you added 6M HI . Does this mistake effect your procedure or not? Explain clearly.arrow_forward
- What is the role of Glycerin in the assay of Boric Acid?I. The reagent prevents the formation of pink color earlier than the expected endpoint.II. The reagent allows the reaction of protons from the acid sample with the hydroxyl ions from the titrant.III. It enhances the water solubility of the difficultly soluble boric acid. a. I, II & III b. II & III c. I & II d. I only e. I & IIIarrow_forwardBaeyer’s test Add a few drops of Baeyer’s Reagent to a test tube containing 0.5 mL of hexane. Baeyer’s reagent is prepared by dissolving potassium permanganate in water to make a 1% solution and making it alkaline by the addition of either sodium carbonate or potassium hydroxide Cover and mix gently Is there an expected color change?arrow_forwardMola and Rity were tasked to analyze the acidity of a 10.0-mL fermented milk sample diluted with water in a 250-mL volumetric flask. Mola obtained a 15.0-mL aliquot of this solution, diluted it to 50.0 mL, and then titrated this diluted sample. Titration data were given to Rity and she found out that the 50.0-mL diluted sample contains 0.21 mmol of lactic acid. MW: Lactic Acid (90.08) Follow proper significant figure rulesa. What is the lactic acid concentration (in M) of the titrated sample? b. Determine the lactic acid content (in M) of the 10.0-mL fermented milk sample. c. What is the lactic acid concentration (in %w/v) of the 10.0-mL fermented milk sample?arrow_forward
- Mola and Rity were tasked to analyze the acidity of a 10.0-mL fermented milk sample diluted with water in a 250-mL volumetric flask. Mola obtained a 15.0-mL aliquot of this solution, diluted it to 50.0 mL, and then titrated this diluted sample. Titration data were given to Rity and she found out that the 50.0-mL diluted sample contains 0.21 mmol of lactic acid. MW: Lactic Acid (90.08) a. What is the lactic acid concentration (in M) of the titrated sample? b. Determine the lactic acid content (in M) of the 10.0-mL fermented milk sample. c. What is the lactic acid concentration (in %w/v) of the 10.0-mL fermented milk sample?arrow_forward1.Describe other melting point apparatus that are available for routine analysis. 2. Explain the differences in melting point of these test compounds by relating with their structures and intermolecular or intramolecular forces of attraction: a. benzoic acid and salicylic acid b. p-aminobenzoic acid and salicylic acid c. oxalic acid and succinic acid d. succinic acid and malic acidarrow_forwardExplain the suitability of using phenolphthalein indicator in the assay of acetic acid.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





