
(a)
Interpretation: Theacid base reaction of benzoic acid with sodium hydroxide needs to be explained.
Concept Introduction:Bronsted and Lowry purposed the Bronsted-Lowry acid-base theory. It states that acid can give
(b)
Interpretation: The acid, base, conjugated acid and conjugated base in the reaction of benzoic acid with sodium hydroxide needs to be determined.
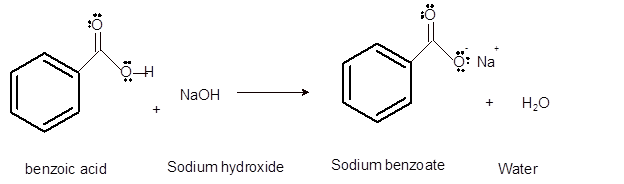
Concept Introduction:Bronsted and Lowry purposed the Bronsted-Lowry acid-base theory. It states that acid can give
(c)
Interpretation: The solubility of benzoic acid and its conjugated base in diethyl ether and water needs to be explained.
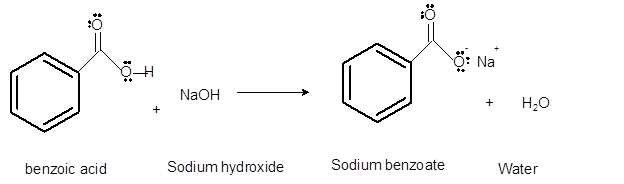
Concept Introduction:Bronsted and Lowry purposed the Bronsted-Lowry acid-base theory. It states that acid can give
Want to see the full answer?
Check out a sample textbook solution
Chapter 5 Solutions
EBK EXPERIMENTAL ORGANIC CHEMISTRY: A M
- In this lab you used acid/base extraction to remove acidic impurities, how could basic impurities be removed through extraction? Imagine the two compounds below are both dissolved in ethyl acetate (a standard organic solvent for extractions), create a diagram/flow chart that outlines steps that could be taken to separate them through acid/base extraction and recover each compound in its neutral Be sure to track when each compound is in the organic or aqueous layer.arrow_forwardthe eugenol and water distillate you collect is apt to be cloudy, particularly the first part of this distillate. Explain why.arrow_forwardWrite the reaction involved in Ferrox Test. a. What is the species responsible? b. Why is phenol negative in Ferrox Test? Based on the theoretical result, what is the order of reactivity of primary, secondary, and tertiary alcohols in the Lucas Test? a. Lucas Reagent contains ZnCl2 in HCl. What is the role of ZnCl2? What reagents are used in the esterification of Alcohols and Phenols? a. Write the reaction involved in Primary Alcohol (Ethanol) and Acetyl Chloride b. Write the reaction involved in Phenol and Acetyl Chloride What is the purpose of the Chromic acid test? a. What are the reagents used? b. Write oxidation reaction of Primary Alcohols and Secondary Alcoholsarrow_forward
- In this experiment a mixture of approximately equal amounts of three compounds - benzoic acid, 2-naphthol, and hydroquinone dimethyl ether - will be separated by a series of liquid-liquid extractions and purified by recrystallization. The first two compounds are acidic (but of considerably different acid strengths) and the third is neutral. Discuss the purification technique used in this experiment, Liquid-Liquid extraction, how does it work? Which core principle does it rely on? (acid-base chemistry)- expand on this. How is acid-base chemistry used to extract compounds? To explain your answer, make sure to use the pKa's of the compounds in your discussion for evidence of how it works.arrow_forwardIf you wish to extract aqueous acetic acid into hexane, is it more effective to adjust the aqueous phase to pH 3 or pH 8? Explainarrow_forwardOn a separate (stapled) paper, outline a separation scheme for isolating benzoic acid from the reaction mixture. (similar to the one in Technique 2, Figure 2.1, Pavia).arrow_forward
- Write the reaction involved in Ferrox Test. a. What is the species responsible? b. Why is phenol negative in Ferrox Test? Based on the theoretical result, what is the order of reactivity of primary, secondary, and tertiary alcohols in the Lucas Test? a. Lucas Reagent contains ZnCl2 in HCl. What is the role of ZnCl2?arrow_forwarda) Write down the products that will occur when you withdraw HBr from 2-bromine-3-methyl butane in a basic medium, State the reaction conditions. Show which product is the main product. b) Does the main product show the geometric isomer, so please write together. If it shows write the isomers. c) Write the product that will be formed when the main product reacts with KMnO4 in a basic environment in cold.arrow_forwardWhat is the order of reactivity of SN1 and SN2 of n-butyl chloride, n-butyl bromide, sec-butyl chloride, tert-butyl chloride and crotyl chloride. Why? Sn2- with NaI/acetone and Sn1- with AgNO3/ethanolarrow_forward
- 1. Why are the acidic extracts and the basic extracts cooled before neutralization during Acid/Base extraction experiment? 2. Why was the water used for washing the precipitates on the funnels specified to be cold during Acid/Base extraction experiment.arrow_forwardSeparating a mixture of equal parts benzoic acid, 2- naphthol and 1,4 - dimethoxybenzene. 1. Explain why base was needed to separate the components of the mixture and why acid was needed to obtain the final product. 2. Which components of the mixture were separated at each stage of the extraction procedure? Explain, making sure to refer to pKa data. 3. Why does foaming occur when acid is added to the bicarbonate extract? Explain with chemical equations.arrow_forwardMelting point range Recrystallized Phenacetin 95-100 Recrystallized Phenacetin + Reference Phenacetin 110-115 Recrystallized Benzil 92-95 Recrystallized Benzil + Reference Benzil 94-98 Questions: 1. What does your melting point say about the purity of your phenacetin sample? Justify your answer using the characteristics of mixed melting point. 2. What does your melting point say about the purity of your phenacetin sample? Justify your answer using the characteristics of melting point. 3. What are two characteristics of a good recrystallization solvent for a given compound? 4. Given a solid sample that contains impurities, how would you determine which solvent to use for a recrystallization? Describe how you would do this in the laboratory. 5. Clearly explain the difference between a recrystallization and a precipitation.arrow_forward
 Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
