
(a)
Interpretation: The 50 % ionization of benzoic acid at pH 4.2 needs to be determined if the pKa of benzoic acid is 4.2.
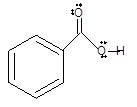
Concept Introduction: Bronsted and Lowry purposed the Bronsted-Lowry acid-base theory. It states that acid can give
(b)
Interpretation: The reason of precipitation of benzoic acid needs to be explained if the pH of an aqueous solution of benzoate ion is lowered to pH 7 by adding acid.
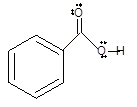
Concept Introduction:Bronsted and Lowry purposed the Bronsted-Lowry acid-base theory. It states that acid can give
Want to see the full answer?
Check out a sample textbook solution
Chapter 5 Solutions
EBK EXPERIMENTAL ORGANIC CHEMISTRY: A M
- 2. The standard Winkler method uses sodium iodide and sodium azide to be added at the same time as sodium hydroxide. What is the purpose of sodium azide? 3. Chlorinated tap water contains both chlorine (Cl2) and hypochlorite (OCl-) does the presence of these species affect DO results? Write equations to represent their individual interactions.arrow_forwardBaeyer’s test Add a few drops of Baeyer’s Reagent to a test tube containing 0.5 mL of hexane. Baeyer’s reagent is prepared by dissolving potassium permanganate in water to make a 1% solution and making it alkaline by the addition of either sodium carbonate or potassium hydroxide Cover and mix gently Is there an expected color change?arrow_forwardWhich statement about the determination of the percentage purity of benzoic acid is correct? * A- Only sodium hydroxide solution can be used as the titrant in the volumetric analysis of benzoic acid. B- Analysing a benzoic acid sample that still contains some hydrochloric acid may yield an analyte percentage that is less than expected. C- Analysing a wet benzoic acid sample may yield an analyte percentage that is more than expected. D- All these statements are correct. E- None of these statements are correct.arrow_forward
- 1. Based on your knowledge on pKa, pH and ionisation describe a method whereby you can separate a solution containing: Sodium bicarbonate and ascorbic acid. 2. Briefly discuss why water is the liquid of choice in (1) car cooling systems and (2) in houses’ central heating systems. 3. Explain how to prepare standard solutions (5 ml each) of NaCl with the following concentrations: 10, 20, 30, 50, 70 ppm. The stock solution has a concentration of 0.05% w/v.arrow_forwardThe standardization of same titrant during the determination of BOD5 of water sample was done separately for Day 1 and Day 5. Upon standardization, it turned out that the concentration of the titrant in Day 1 is higher than the concentration of the same batch of titrant in Day 5. How would you explain this discrepancy? The water used to prepare the Na2S2O3 solution was not boiled resulting to proliferation of bacteria. Too much starch indicator was added in the conical flask. Sulfuric acid was added first before potassium iodide. There were bubbles at the tip of the burette during titration.arrow_forwardYou are conducting a biochemical experiment with an enzyme that has optimal activity at pH = 6.50. You decide to use carbonate (pKa1 = 6.38, pKa2 = 10.30) as the buffer to keep the pH stable throughout the enzymatic reaction. (Recall that the formula for carbonic acid is H2CO3.) You prepare a 0.4 M solution of carbonate buffer at pH = 10.50. Calculate the concentrations of the major carbonate species in your solution. Show your calculations.arrow_forward
- An aqueous solution of benzoic acid and sodium benzoate has a pH of 5.1. The dissociation constant of benzoic acid is 6.5 x 10^-5. What is the pKa of benzoic acid? What is the molar concentration of sodium benzoate (conjugate base)? What is the molar concentration of benzoic acid (weak acid)?arrow_forward1. What are some precautions that should be observed when collecting samples for DO determination, to ensure the amount of oxygen in the sample is representative of the DO at the site? 2. The standard Winkler method uses sodium iodide and sodium azide to be added at the same time as sodium hydroxide. What is the purpose of sodium azide? 3. Chlorinated tap water contains both chlorine (Cl2) and hypochlorite (OCl-) does the presence of these species affect DO results? Write equations to represent their individual interactions. 4. What effect would nitrate (NO2-), a common constituent of polluted water, have on DO results? Write a balanced equation for its interaction. 5. What is meant by seawater intrusion in drinking water supplies? How does this happen? What are some consequences of these phenomena?arrow_forwardSuppose your colleague was relying on receiving your purified product in order to prepare a dilute aqueous standard solution of benzoic acid e.g. for a titration. She takes 160 mg of your benzoic acid and dissolves it in just enough water so the solution exactly fills a 250 mL volumetric flask. Given that the acid dissociation constant of benzoic acid Ka = 6.3 x 10-5, calculate the pH of her resulting solution.arrow_forward
- For acid-base extractions: Explain what result would you expect if you accidentally extracted the mixture in the reverse order (HCl, NaOH, NaHCO3)? [instead of using NaHCO3 first, NaOH second, and then HCl third]arrow_forwardIn this experiment a mixture of approximately equal amounts of three compounds - benzoic acid, 2-naphthol, and hydroquinone dimethyl ether - will be separated by a series of liquid-liquid extractions and purified by recrystallization. The first two compounds are acidic (but of considerably different acid strengths) and the third is neutral. Discuss the purification technique used in this experiment, Liquid-Liquid extraction, how does it work? Which core principle does it rely on? (acid-base chemistry)- expand on this. How is acid-base chemistry used to extract compounds? To explain your answer, make sure to use the pKa's of the compounds in your discussion for evidence of how it works.arrow_forwardThe NaOH titrant in this experiment was prepared to be approximately 0.1 M and then was standardized to determine its exact concentration. What possible reasons could there be for not simply weighing the solid NaOH, dissolving to a known volume and calculating its molarity?arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





