
(a)
To determine: The method of conversion of
Interpretation: The method of conversion of
Concept introduction: NBS is N-bromosuccinimide. It is used as an alternative for
(a)
Answer to Problem 6.30SP
The conversion of
Explanation of Solution
The conversion of
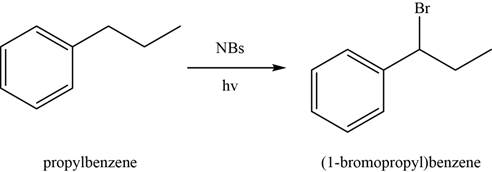
Figure 1
In the above reaction,
(b)
To determine: The method of conversion of
Interpretation: The method of conversion of
Concept introduction: NBS is N-bromosuccinimide. It is used as an alternative for
(b)
Answer to Problem 6.30SP
The conversion of
Explanation of Solution
In first step,
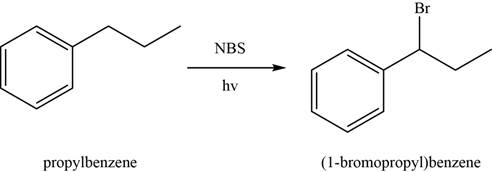
Figure 2
In the second step,
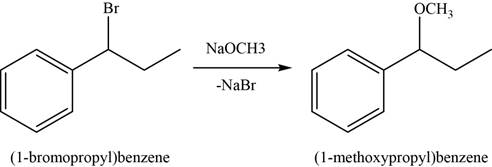
Figure 3
(c)
To determine: The method of conversion of
Interpretation: The method of conversion of
Concept introduction: NBS is N-bromosuccinimide. It is used as an alternative for
(c)
Answer to Problem 6.30SP
The conversion of
Explanation of Solution
In first step,
In the second step,
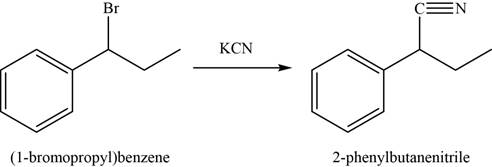
Figure 4
Want to see more full solutions like this?
Chapter 6 Solutions
Organic Chemistry (9th Edition)
- Propose an efficient synthesis for the following transformation: 1-butyne to trans-3,4-dibromohexaneThe transformation above can be performed with some reagent or combination of the reagents listed below. Give the necessary reagent(s) in the correct order, as a string of letters (without spaces or punctuation, such as “EBF”). If there is more than one correct solution, provide just one answer. A B C Br2 NaNH2 Na, NH3 (l) D E F dilute H2SO4 H2, Lindlar's catalyst MeI G H I 1) BH3·THF2) H2O2, H2O 1) xs NaNH22) H2Oarrow_forwardShow how you would convert toluene to 3,5-dibromotoluene in good yieldarrow_forward4. using retrosynthetic analysis, determine which compounds could lead to the alkene shows below in a single steparrow_forward
- Find the product by writing down the mechanism of the reaction given below.arrow_forward1.Show the Elimination reaction of 2-bromopentane. 2.What contributed to formation of minor and major product?arrow_forwardConsider the reaction of the cyclopentanone derivative shown below. Draw the structure for the major product formed in this reaction. The cyclopentanone ring has been provided as a starting point.arrow_forward
- 3) While we expect to get one major product, this reaction could potentially have some issues with formation of multiple products. What is the relationship between the two major products that you might expect for the reaction of E-stilbene and dimethyl fumarate? Why will this not matter when you look at the NMR? 4) In addition to the issue raised in prelab Q3, 2,3-dihydrofuran and styrene will also give give two additional major products – what is their relationship, and will this matter when you look at the NMR?arrow_forwardGive a detailed reaction mechanism for the reaction expected to occur when 2-bromo-2-methylpentane is heated with sodium methoxide. Draw clear structural formulas of all relevant species and use curved arrows to represent electron flow. Also indicate which step is likely to be rate-determining. The answer you sent before will be used for this question.arrow_forwardWrite what the products A, B, C, D, E, F, G, H, I, J and K are in the reactions below.arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
