
Organic Chemistry (9th Edition)
9th Edition
ISBN: 9780321971371
Author: Leroy G. Wade, Jan W. Simek
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 6.2, Problem 6.3P
For each of the following compounds,
- A. give the IUPAC name
- B. give the common name (if possible).
- C. classify the compound as a methyl, primary, secondary, or tertiar
- a. (CH3)2CHCH2C1
- b. (CH3)3CBr
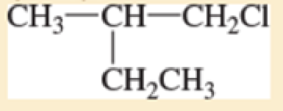
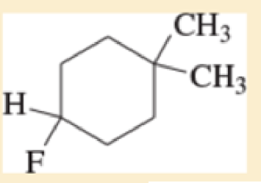
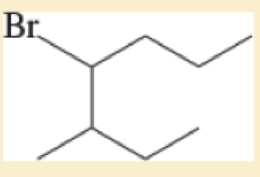
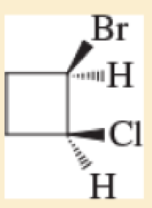
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
The melting points and boiling points of two isomeric alkanes are asfollows: CH3(CH2)6CH3, mp = −57 °C and bp = 126 °C; (CH3)3CC(CH3)3,mp = 102 °C and bp = 106 °C.
Explain why one isomer has a lower melting point but higher boiling point.
The skeletal line formula for a branched alkene is shown below. (i) What is the molecular formula of this compound? (ii) How many carbon atoms are in the longest chain, ignoring the double bond? (iii) What is the longest chain incorporating both carbons of the double bond? (iv) How many substituents are on this chain? (v) Give the IUPAC name for this compound. [6]
Which compound will give a positive test with Br2/CH3Cl, a positive test with HCl/ZnCl2, and a positive test with CrO3/H2SO4?
Chapter 6 Solutions
Organic Chemistry (9th Edition)
Ch. 6.1 - Classify each compound as an alkyl halide, a vinyl...Ch. 6.2 - Give the structures of the following compounds. a....Ch. 6.2 - For each of the following compounds, A. give the...Ch. 6.3E - Prob. 6.4PCh. 6.4 - Prob. 6.5PCh. 6.5A - For each pair of compounds, predict which compound...Ch. 6.5B - Prob. 6.7PCh. 6.6B - Prob. 6.8PCh. 6.6B - The light-initiated reaction of...Ch. 6.6B - Show how free-radical halogenation might be used...
Ch. 6.7 - Prob. 6.11PCh. 6.7 - Prob. 6.12PCh. 6.8 - Prob. 6.13PCh. 6.9 - Predict the major products of the following...Ch. 6.9 - Prob. 6.15PCh. 6.10A - Prob. 6.16PCh. 6.11A - When diethyl ether (CH3CH2OCH2CH3) is treated with...Ch. 6.11B - Prob. 6.18PCh. 6.11B - For each pair of compounds, state which compound...Ch. 6.12 - Prob. 6.20PCh. 6.12 - Under appropriate conditions...Ch. 6.13 - Propose an SN1 mechanism for the solvolysis of...Ch. 6.13B - Prob. 6.23PCh. 6.13B - 3-Bromocyclohexene is a secondary halide, and...Ch. 6.15 - Prob. 6.25PCh. 6.15 - Prob. 6.26PCh. 6.16 - For each reaction, give the expected substitution...Ch. 6.16 - Prob. 6.28PCh. 6.16 - Prob. 6.29PCh. 6 - Prob. 6.30SPCh. 6 - Draw the structures of the following compounds. a....Ch. 6 - Give systematic (IUPAC) names for the following...Ch. 6 - Prob. 6.33SPCh. 6 - Predict the compound in each pair that will...Ch. 6 - Prob. 6.35SPCh. 6 - Give two syntheses for (CH3)2CHOCH2CH3, and...Ch. 6 - Prob. 6.37SPCh. 6 - Prob. 6.38SPCh. 6 - Chlorocyclohexane reacts with sodium cyanide...Ch. 6 - Give the substitution products expected from...Ch. 6 - Prob. 6.41SPCh. 6 - Prob. 6.42SPCh. 6 - Two of the carbocations in Problem6-42 are prone...Ch. 6 - Prob. 6.44SPCh. 6 - Predict the products of the following SN2...Ch. 6 - Prob. 6.46SPCh. 6 - Strawberry growers have used large quantities of...Ch. 6 - A solution of pure (S)-2-iodobutane ([]=+15.90) in...Ch. 6 - Prob. 6.49SPCh. 6 - Give a mechanism to explain the two products...Ch. 6 - Prob. 6.51SPCh. 6 - Because the SN1 reaction goes through a flat...Ch. 6 - Prob. 6.53SPCh. 6 - Furfuryl chloride can undergo substitution by both...Ch. 6 - Prob. 6.55SPCh. 6 - The following reaction takes place under...Ch. 6 - Propose mechanisms to account for the observed...Ch. 6 - Prob. 6.58SPCh. 6 - Prob. 6.59SP
Additional Science Textbook Solutions
Find more solutions based on key concepts
Label each statement about the polynucleotide ATGGCG as true or false. The polynucleotide has six nucleotides. ...
General, Organic, & Biological Chemistry
Write the electron configurations far each of the following elements: (a) Sc. (b) Ti. (c) Cr. (d) Fe. (e) Ru
Chemistry by OpenStax (2015-05-04)
Give the IUPAC name for each compound.
Organic Chemistry
141. Design a device that uses as electrochemical cell to determine amount of
in a sample water Describe, in...
Chemistry: Structure and Properties (2nd Edition)
45. Calculate the mass of nitrogen dissolved at room temperature in an 80.0-L home aquarium. Assume a total pre...
Chemistry: Structure and Properties
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Dr. Daniel discovered three new saturated hydrocarbon compounds (compounds A,B, and C) and wanted to determine their physical and chemical properties. Compound A and B are optically inactive and vice versa for compound C. When these three compounds undergoes oxidation process, it gives negative results. Besides, it also not decolorize the bromine water during chemical reaction. i. Draw the possible skeletal formula for compounds A and B containing at least two substituents. ii. Draw the line-wedge-dash-projection based on diastereomer of compound C and label their optically active site . iii. Identify the IUPAC nomenclature name for compounds A, B and C. Label the carbon and hydrogen classification for compounds A, B, and C. iv. List two (2) physical properties and two (2) chemical properties for compounds B. v. Discuss the sequence of compounds A, B, and C according to their boiling point. vi. Predict the complete chemical reaction for the combustion of compound Barrow_forwardin the structure, replace the "A" labels with groups to satisfy all of these conditions:- Put a Br anti to the methyl group already drawn in.- Put a hydroxyl group (OH) gauche to F and anti to Cl.- Put a benzene ring anti to F. (You will need to replace the "A" with a carbon when you do this.)arrow_forwardWhy is the carbon bonded to the methyls numbered 2 and not 1?arrow_forward
- What is the purpose of Bromine in the CCl4 and Ignition Test? What is the reason behind the yellow sooty flame when a compound has a high degree of unsaturation? Show a reaction of complete combustion and incomplete combustionarrow_forward(2) Show the complete reaction of the following. Draw the structure and give the IUPAC and common naming. b. Reaction of 1-Butanethiol and 2- methyl-2-propanethiolarrow_forwardOrganic Chemistry Incorrect Separation Scheme Cannot be hand-drawn *see attached for the incorrect separation scheme provided. The top of the separation scheme shows what other compound is mixed with your molecule (2,6-dimethyloct-2-ene). Assume for the purposes of this assignment that both compounds are solid at room temperature. Also assume that both compounds are soluble in ether, except ionic compounds. The goal of the separation is the isolate each of the two compounds from the mixture. Below the incorrect separation scheme write a discussion of this incorrect scheme identifying all of the mistakes in the separation scheme. Keep in mind that there will be more than one mistake in the scheme. For each mistake, give a detailed, scientific explanation of why it is incorrect.arrow_forward
- here's the reaction to be used for reference: C2H4 + H2 --> C2H6 1. What type of reaction is involved in C2H4 + H2 --> C2H6? 2. What is C2H4 in the reaction? 3. What is the name of the product in the reaction? a. ethane b. ethene c. ethynearrow_forwardWhich of the statements about the hydration of alkenes and alkynes are true? * A- Both alkenes and alkynes are nucleophiles in addition reactions. B- Alkenes that are sensitive to rearrangement can be hydrated indirectly using Hg²⁺ as the hydration catalyst followed by reduction. C- The hydration of alkenes and alkynes both involves one nucleophilic addition step. D- All these statements are true. E- None of these statements are true.arrow_forwarda) what important physical property of alcohols differentiate them from the other functional groups? b) is the O-H bond strengthened or weakened by this effect? (broad band spectrums)arrow_forward
- Draw all possible constitutional isomers for C2H6O and give common and IUPAC names for each structure.arrow_forwardIndicate whether each statement is true or false. (a) Butanecontains carbons that are sp2 hybridized. (b) Cyclohexaneis another name for benzene. (c) The isopropyl group containsthree sp3-hybridized carbons. (d) Olefin is anothername for alkyne.arrow_forwarda. Draw the structures and give the common and systematic names for alkynes with molecular formula C7H12. Ignore stereosiomers. (Hint: There are 14.) b. How many would there be if stereoisomers are included?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co
Nomenclature: Crash Course Chemistry #44; Author: CrashCourse;https://www.youtube.com/watch?v=U7wavimfNFE;License: Standard YouTube License, CC-BY