
EP ORGANIC CHEMISTRY -MOD.MASTERING 18W
9th Edition
ISBN: 9780136781776
Author: Wade
Publisher: PEARSON CO
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 6, Problem 6.45SP
Predict the products of the following SN2 reactions
- a. CH3CH2ONa+CH3CH2Cl→
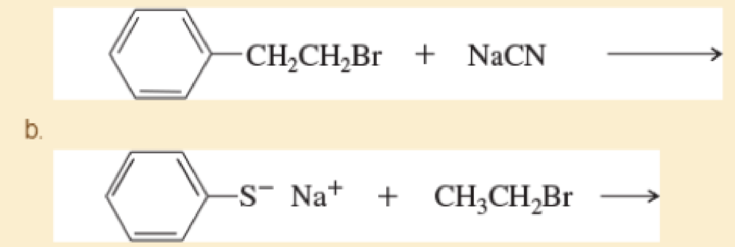
c.
d. CH3(CH2)8CH2Cl+Na+-:≡CH→
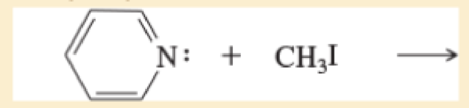
e.
f. (CH3)3C—CH2CH2Br+excessNH3→
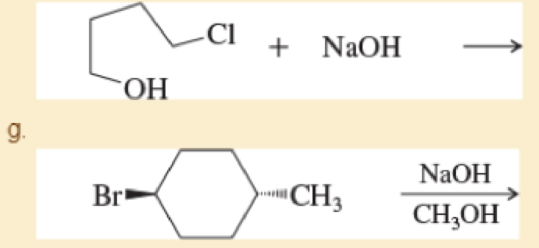
h.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
From the table of reagents shown below, show how you synthesize the product from the given reactant.
Br
Reagents available
a. NBS, (PhCO2)2 h. CH3 Cl, AIC13
CUCN
0.
b. Br2, FeBr3
i. CH3 CH2 Br
р. НзО*, д
с. Вг2, Н+
j. CH3O¯, A
q. CH; CH2 COCI, AICI3
r.
k. CH3 COCI, AICI3
H2NNH2,
OH, A
d. OH-
e. NaOH(s), A
1. Mg, Et20
s. H2, Pd/C
f. HNO3, H,SO4
m. CO2(s) then H3O+ t. H3PO2
g. H2 CrO4
n. HONO 0°C
u. Cl2, FeCl3
(Enter the letter(s) of the reagent(s) needed in the box, in the order that they must be used. No more than two steps are required to
perform the synthesis. Note that a retrosynthetic arrow is used, and therefore the reactant is shown on the right.)
Answer:
select reagents:
cyclopentanone
Reagents
a. C6H5CHO
b. NaOH, ethanol
c. Pyrrolidine, cat. Hj.
d. H₂C=CHCN
e. H3O+
f. LDA
g.
A) bg
EtOC(=O)CO₂Et
h.
i.
B) ka
k.
I.
m.
O:
BrCH₂CH=CH₂
NatOEt, ethanol
Br₂, H+
K+ t-BUO™
CH2(CO2Et)z
heat
C) jh
CO₂Et
D) Im
6. Provide the reagents or the product for the following reactions.
Br
a
b.
HO
он
C.
PB13
Chapter 6 Solutions
EP ORGANIC CHEMISTRY -MOD.MASTERING 18W
Ch. 6.1 - Classify each compound as an alkyl halide, a vinyl...Ch. 6.2 - Give the structures of the following compounds. a....Ch. 6.2 - For each of the following compounds, A. give the...Ch. 6.3E - Prob. 6.4PCh. 6.4 - Prob. 6.5PCh. 6.5A - For each pair of compounds, predict which compound...Ch. 6.5B - Prob. 6.7PCh. 6.6B - Prob. 6.8PCh. 6.6B - The light-initiated reaction of...Ch. 6.6B - Show how free-radical halogenation might be used...
Ch. 6.7 - Prob. 6.11PCh. 6.7 - Prob. 6.12PCh. 6.8 - Prob. 6.13PCh. 6.9 - Predict the major products of the following...Ch. 6.9 - Prob. 6.15PCh. 6.10A - Prob. 6.16PCh. 6.11A - When diethyl ether (CH3CH2OCH2CH3) is treated with...Ch. 6.11B - Prob. 6.18PCh. 6.11B - For each pair of compounds, state which compound...Ch. 6.12 - Prob. 6.20PCh. 6.12 - Under appropriate conditions...Ch. 6.13 - Propose an SN1 mechanism for the solvolysis of...Ch. 6.13B - Prob. 6.23PCh. 6.13B - 3-Bromocyclohexene is a secondary halide, and...Ch. 6.15 - Prob. 6.25PCh. 6.15 - Prob. 6.26PCh. 6.16 - For each reaction, give the expected substitution...Ch. 6.16 - Prob. 6.28PCh. 6.16 - Prob. 6.29PCh. 6 - Prob. 6.30SPCh. 6 - Draw the structures of the following compounds. a....Ch. 6 - Give systematic (IUPAC) names for the following...Ch. 6 - Prob. 6.33SPCh. 6 - Predict the compound in each pair that will...Ch. 6 - Prob. 6.35SPCh. 6 - Give two syntheses for (CH3)2CHOCH2CH3, and...Ch. 6 - Prob. 6.37SPCh. 6 - Prob. 6.38SPCh. 6 - Chlorocyclohexane reacts with sodium cyanide...Ch. 6 - Give the substitution products expected from...Ch. 6 - Prob. 6.41SPCh. 6 - Prob. 6.42SPCh. 6 - Two of the carbocations in Problem6-42 are prone...Ch. 6 - Prob. 6.44SPCh. 6 - Predict the products of the following SN2...Ch. 6 - Prob. 6.46SPCh. 6 - Strawberry growers have used large quantities of...Ch. 6 - A solution of pure (S)-2-iodobutane ([]=+15.90) in...Ch. 6 - Prob. 6.49SPCh. 6 - Give a mechanism to explain the two products...Ch. 6 - Prob. 6.51SPCh. 6 - Because the SN1 reaction goes through a flat...Ch. 6 - Prob. 6.53SPCh. 6 - Furfuryl chloride can undergo substitution by both...Ch. 6 - Prob. 6.55SPCh. 6 - The following reaction takes place under...Ch. 6 - Propose mechanisms to account for the observed...Ch. 6 - Prob. 6.58SPCh. 6 - Prob. 6.59SP
Additional Science Textbook Solutions
Find more solutions based on key concepts
The active ingredient in Tylenol and a host of other over-the-counter pain relievers is acetaminophen (C8H9NO2)...
Chemistry: Atoms First
Q2. Which statement best defines chemistry?
a. The science that studies solvents, drugs, and insecticides
b. Th...
Introductory Chemistry (6th Edition)
Draw a Lewis structure for each covalent molecule. a. HBr b. CH3F c. H2O2 d. N2H4 e. C2H6 f. CH2Cl2
Principles of General, Organic, Biological Chemistry
Determine [OH], [H+], and the pH of each of the following solutions. a. 1.0 M KCl b. 1.0 M KC2H3O2
Chemistry
2. Why shouldn’t you work in a laboratory by yourself?
The Organic Chem Lab Survival Manual: A Student's Guide to Techniques
Give the IUPAC name for each compound.
Organic Chemistry
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Which of the following compounds can be used to synthesize ethanoicpropanoic anhydride (CH;COOOCCH½CH3)? A. CH;COCI+ CH;CH2COO`Na* → B. CH;COOH +heat → C. CH3CH2CH2COCI + CH;COO Na* → D. A and Carrow_forwardFrom the table of reagents shown below, show how you synthesize the product from the given reactant. OCH₂CH3 OH a. NBS, (PhCO₂)2 b. Br2, FeBr3 c. Br₂, H+ d. OH e. NaOH(s), A f. HNO3, H₂SO4 g. H₂ CrO4 Reagents available h. CH3 CI, AICI, o. CuCN i. CH3 CH₂ Br p. H₂O+, A j. CH₂O, A q. CH3 CH₂ COCI, AICI, k. CH3COCI, AICI3 r. H₂NNH₂, OH, A 1. Mg, Et₂ O s. H₂, Pd/C m. CO₂ (s) then H3O+ t. H₂PO₂ n. HONO 0°C u. Cl₂, FeCl3 (Enter the letter(s) of the reagent(s) needed in the box, in the order that they must be used. No more than two steps are required to perform the synthesis. Note that a retrosynthetic arrow is used, and therefore the reactant is shown on the right.) Answer:arrow_forwardComplete the following reaction scheme. 1. DIBALH 2. H3O+ TMSCI Pyridine 1. LIAIH(t-BuO)3 SOCI2 2. H3O+ CrO3 H3O+arrow_forward
- In which compound is the halogen substituted most rapidly by aq hydroxide ions? a. CH3CH2CH2CH2Cl b. (CH3)3CI c. (CH3)3CCl d. CH3CH2CH2CH2Iarrow_forwardOCH3 CH3 c-H Determine the product formed when the given compound is reacted with the reagent given below Choose from the following: A. В. C. D. E. OCH3 OCH3 OCH; CH2OCH3 CH,OH HO HO HO HO CH; `CH3 CH3 `CH;OH CH;´ `CH2OH CH;´ `CH;OH CH3 `CH;OH Reagent: excess H2, metal catalystarrow_forwardCH2 CH; e. CH2=CHCCH3 с. a. CH;CHCH=CH2 ČH3 ČH3 CH3 CH3 d. f. b. CH3CHCH2CH=CH2 CH3 What is the major product obtained from the reaction of HBr with each of the followingarrow_forward
- Which undergoes most rapid reaction on treatment with AICI3 and an acid halide a. b. C. d. benzene toluene chlorobenzene 1,4-dichlorobenzenearrow_forwardStarting Materials CH3B CH3CH2BR CH3CH2CH,Br C6H5Br Reagents Mg, ether 1. a. 2. 13CH2-13C-OH b. 13CO2 then H3O* 3. LIAIH4 then H3o* PBr3 CO2 then H30* C. 4. d. 5. C6HsCH2Br е. Select a starting material and a series of reagents necessary to synthesize this compound in as few steps as possible. Enter your selection as a number for the starting material, followed by the letters of the reagents, in the order that you wish to use them, i.e. 3abcf.arrow_forwardDetermine whether A or B reacts faster in an 80% ethanol mixture and provide two reasons as to why. MeO O₂N. A NO₂ MeO O₂N. NO₂arrow_forward
- 6. Provide the structure for the major product in the following reactions. b. P f. OH g. Lia * Oia ملی CI 1. SOCI₂, pyr. 2. to Br 2 1. LIAIH4 (xs) 2. H₂O 1. LIAI(OR) 3H 2. H₂O 2. NH₂ Et₂CuLi 1. EtMgBr (xs) 2. H₂O 1. Mg 2. CO₂ 3. H* 4. SOCI₂, pyr 1. [H], NaBH3CN, EtNH₂ CI pyridinearrow_forwardExplain why each of the following reactions will not proceed as written. [1] LDA + Co2 a. C. [2] CH;CH2I COOH [1] NaOEt b. CH2(CO,Et)2 (CH,CH2),CCH(CO̟Et)2 (2] (CH3CH2),CBrarrow_forwardPredict the reagent(s) needed to produce this product(s). H. O O A B C D E 1. Os04 (catalytic) 2. NMO 1. OsO4, H2O 2. NaIO4 3. Na2S2O3, H₂O 1. KMnO4, OH¯ (warm) 2. H3O+ 1. mCPBA 2. H3O+ mCPBA Donearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
Coenzymes and cofactors; Author: CH15 SWAYAM Prabha IIT Madras;https://www.youtube.com/watch?v=bubY2Nm7hVM;License: Standard YouTube License, CC-BY
Aromaticity and Huckel's Rule; Author: Professor Dave Explains;https://www.youtube.com/watch?v=7-BguH4_WBQ;License: Standard Youtube License